The phaeophycean, fucoid alga,
FCPs have been reported to exhibit a wide variety of bioactivities such as antioxidant, anti-bacterial, anticoagulant, anti-inflammatory, and anti-proliferative activities (Fitton 2011). Fucoidan from the brown seaweed
Bacteria communicate through an extensive array of extracellular signal molecules. Production and secretion of these extracellular signal molecules mediate cell-to-cell communication which coordinates the expression of various genes within the bacterial population and aids in the formation of biofilms in response to specific environmental or physiological conditions, which, in turn, enhances successful infection in humans and in animals (Davies et al. 1998, Wiener-Kronish et al. 2001). The process of sensing individual cells by the accumulation of diffusible, low-molecular-weight signal molecules is known as “quorum sensing” (QS) (Williams et al. 2000). There are two well-defined QS systems identified in
In this study, we used the
>
Chemicals and Caenorhabditis elegans strains
All chemicals were purchased from Sigma-Aldrich, Oakville, Ontario, Canada, unless otherwise stated. The wild type N2 (var. Bristol), mutant (
A powder form of commercially extracted FCP was a kind gift, prepared by Marinova (http://www.marinova.com.au/). The material was derived from
[Appendix 1.] The PCR primers designed for the quorum sensing and virulence gene expression
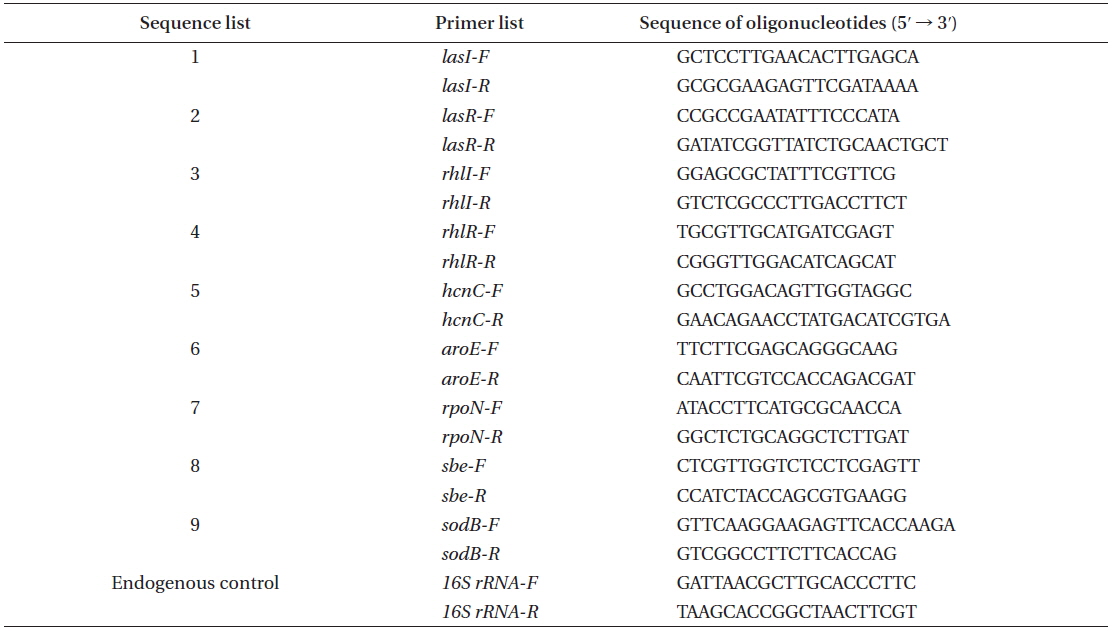
The PCR primers designed for the quorum sensing and virulence gene expression
>
Bacterial strains and growth conditions
The clinical, pathogenic isolate of
>
Testing of antimicrobial susceptibility
The disk diffusion method of the Clinical and Laboratory Standards Institute (CLSI) was used to determine the antimicrobial susceptibility of the
Bacterial culture was streaked on the control and the different concentrations of FCP-treated, LB agar. Single colonies of bacteria were subjected to different motility assays. All the assays were repeated three times, each with three replicates.
A single colony of PA14 bacteria was inoculated into 5 mL of LB media and grown for 15 h at 37°C with aeration. Two microliters of each overnight culture was spotted onto the surface of LB 0.5% agar and SK 0.5% agar plates and then incubated at 37°C overnight.
A single bacterial colony was picked with a straight end loop and inoculated into LB swim agar (0.35% agar). Plates were incubated 8-12 h at 37°C. The diameter of the flagellum-mediated motility generated turbid zone was measured.
A portion of a single bacterial colony was picked with a straight end inoculation loop and stabbed to the bottom of a LB agar plate (1.5% agar). Plates were incubated overnight at 37°C and then for 2 days at room temperature. The growth, at the interface between the agar and the polystyrene plate (radius from the inoculation point), was measured.
>
The effect of FCP on Pseudomonas aeruginosa protease
Protease activity was determined by measuring the ability of culture supernatants to lyse skimmed milk powder. Supernatants from the 18-hour-old culture, grown at 37°C, with constant shaking, were used for the assay. A 100 µL aliquot of
>
Effect of FCP on Pseudomonas aeruginosa alkaline protease
Alkaline protease activity of the supernatant from an overnight bacterial culture in LB broth was determined by adding 0.5 mL of supernatant to 1.5 mL of assay buffer (20 mM Tris-HCl, 1 mM CaCl2 buffer, pH 8.0) which contained 50 mg of hide remazol blue powder (Sigma-Aldrich). Tubes were incubated at 37°C for 1 h with constant shaking; the reaction was stopped by placing the tube on ice. After centrifuging 5 min at 4,000 ×g, the absorbance of the supernatant was measured at 590 nm after 24 and 48 h. The enzyme activity was expressed as units where an increase of 1.0 in the OD590 per milliliter per hour was defined as 1 unit.
>
Effect of FCP on Pseudomonas aeruginosa elastin
The secreted elastase in the supernatant of PA14 was measured using Congo Red as the substrate. The bacterium was grown in LB broth at 37°C for 16 h, centrifuged at 15,000 ×g, at 4°C, for 10 min; 0.5 mL of the supernatant was added to 1 mL of the assay buffer (30 mM Tris buffer, pH 7.2) containing 10 mg of Congo Red (Sigma-Aldrich). The mixture was incubated at 37°C for 6 h with constant shaking. The insoluble substrate was removed by centrifugation at 1,200 ×g for 10 min and the absorbance of the supernatant was measured at 495 nm. Elastase activity was defined as an increase in the OD495 per milliliter of PA14 culture filtrate.
>
Effect of FCP on Pseudomonas aeruginosa pyocyanin
Pyocyanin was extracted from a 24-hour-old
>
Effect of FCP on the production of hydrogen cyanide in Pseudomonas aeruginosa
A bacterial culture was streaked onto tryptic, soya agar medium. Filter paper discs (1.5 cm diameter) were soaked in picric acid solution (2.5 g picric acid, 12.5 g Na2CO3, and 1 L distilled water) and placed on the upper lids of Petri dishes. The dishes were sealed with parafilm and incubated for four days. HCN production was assessed by the presence of a coloured zone around the bacterial lawn and the colour change of the filter paper from yellow to a brown to reddish-brown. Reaction colours were scored as “weak” (i.e., yellow to light brown), “moderate” (i.e., brown) and “strong” (i.e., reddish brown).
>
Quantification of hydrogen cyanide
Bacterial isolates were grown in tryptic soya broth with picric acid solution saturated filter paper strips (10 cm long and 0.5 cm wide) in a hanging position, inside the flask at 28 ± 2°C for 48 h. The sodium picrate in the filter paper was reduced to a reddish compound in proportion to the amount of hydrocyanic acid evolved. The coloured pigment was eluted by placing the filter paper in a clean test tube containing 10 mL of distilled water and measuring absorbance at 625 nm.
>
Quantitation of siderophores
The bacterium was grown in KB broth for 3 days and centrifuged at 2,000 rpm for 10 min. The pH of the supernatant was adjusted to 2.0 with HCl and an equal volume of ethyl acetate was added in a separating funnel, mixed well and the ethyl acetate fraction was collected. This process was repeated three times to recover most of the siderophores from the supernatant. The ethyl acetate fractions were pooled, air-dried and dissolved in 5 mL of ethanol (50%). Five milliliters of ethyl acetate fraction was mixed with 5 mL of Hathway’s reagent (1.0 mL of 0.1 M FeCl3 in 0.1 N HCl to 100 mL of distilled water containing 1.0 mL of potassium ferricyanide). The absorbance for dihydroxyphenol was measured at 700 nm using dihydroxy benzoic acid as a standard. The synthesis of siderophores was expressed as µM mL-1 of culture filtrate.
The relative expressions of quorum sensing genes (i.e.,
The biofilm forming ability of the isolates was observed using polystyrene microtitre plates. Overnight cultures in LB broth were diluted 1 : 100 into fresh LB broth and then 0.1 mL of the freshly inoculated medium was dispensed into a 96-well polystyrene microtitre plate. The plates were incubated at 37°C for 8 h without agitation. Biofilm formation was observed by staining the wells with 10 µL of crystal violet [0.1% (w/v) in water]. After the stain was added, the plates were incubated for a further 15 min at room temperature and then washed thoroughly with distilled water to remove cells and residual dye. Ethanol (95%) was used to elute any crystal violet from the biofilms and the absorbance of the solubilized dye was measured at 590 nm using a microtitre plate reader (BioTek, Winooski, VT, USA).
MNGA medium was used in slow-killing assays to test the efficacy of FCP on PA14-induced killing. Treatment plates were prepared by spreading 10 µL of saturated PA14 culture on the center of the plate. The plates were incubated at 37°C, for 12 h to establish the PA14 lawn.
>
Killing assays with mutant Caenorhabditis elegans worms
Three different immune responsive, functional mutant strains (i.e.,
>
Effect of FCP on immune-response gene expression of Caenorhabditis elegans
About 500 synchronized, young adult worms from each treatment were used in this study. The treatment details are as follows: 1) control (worms cultured on
[Appendix 2.] The immune response primers used in this study
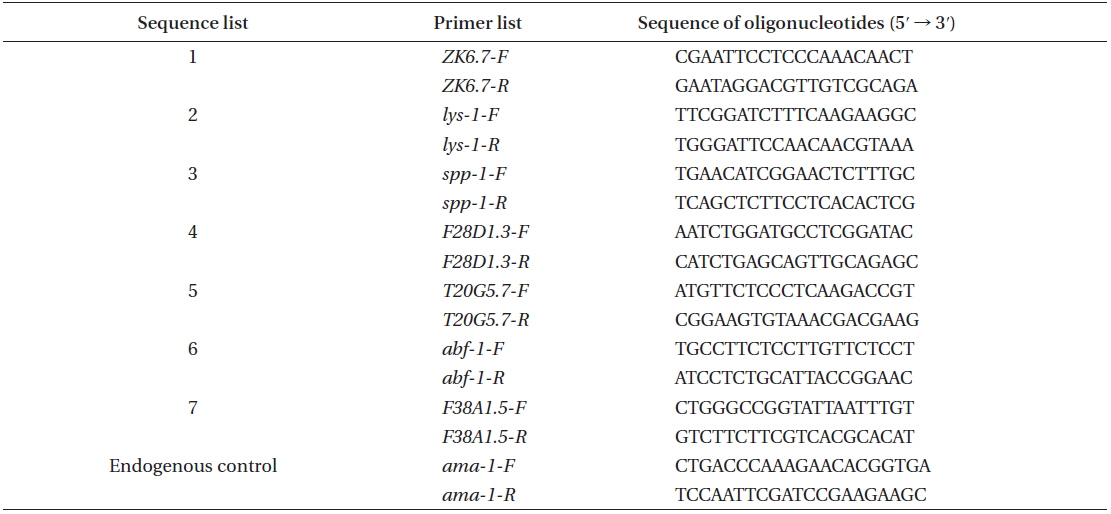
The immune response primers used in this study
Significance of the data was analyzed using COSTAT; p <0.05 was considered to be statistically significant. Data were analyzed using Fisher’s least significant difference test with p ≤ 0.05 using COSTAT statistical software.
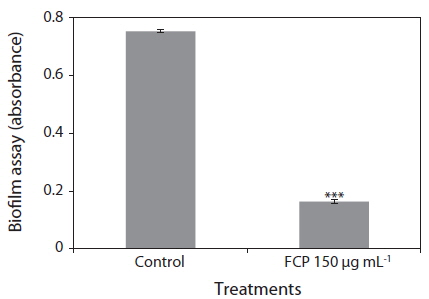
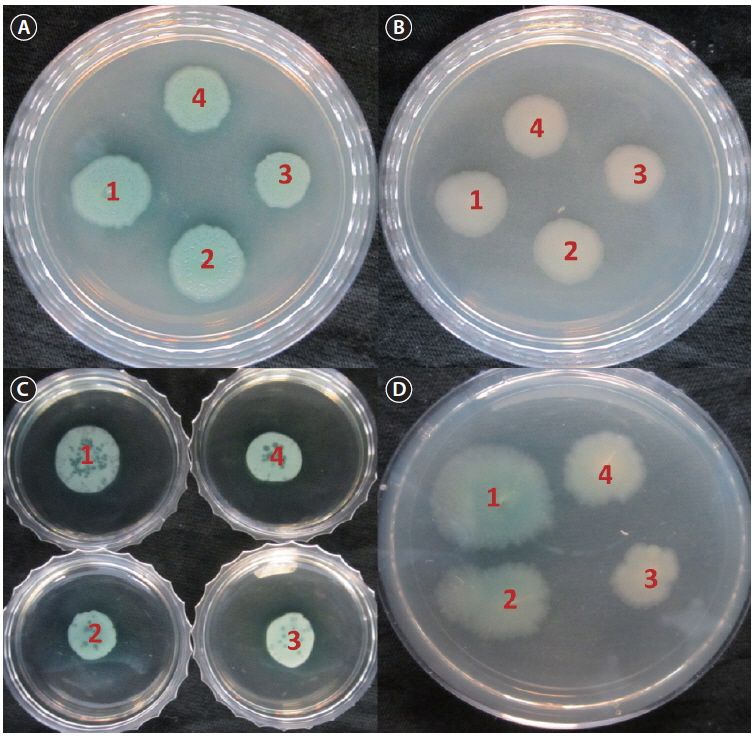
Biofilms act as environmental reservoirs of pathogens; the film provides the organisms with survival advantages in natural environments and increases their virulence. The FCP-treatment reduced the biofilm formation by a factor of 4, as compared to the control (p < 0.0001) (Fig. 1). The direct antimicrobial activity of FCP was tested by placing paper discs with different concentrations of FCP on petri-plates cultured with
>
FCP inhibited Pseudomonas aeruginosa toxic metabolites
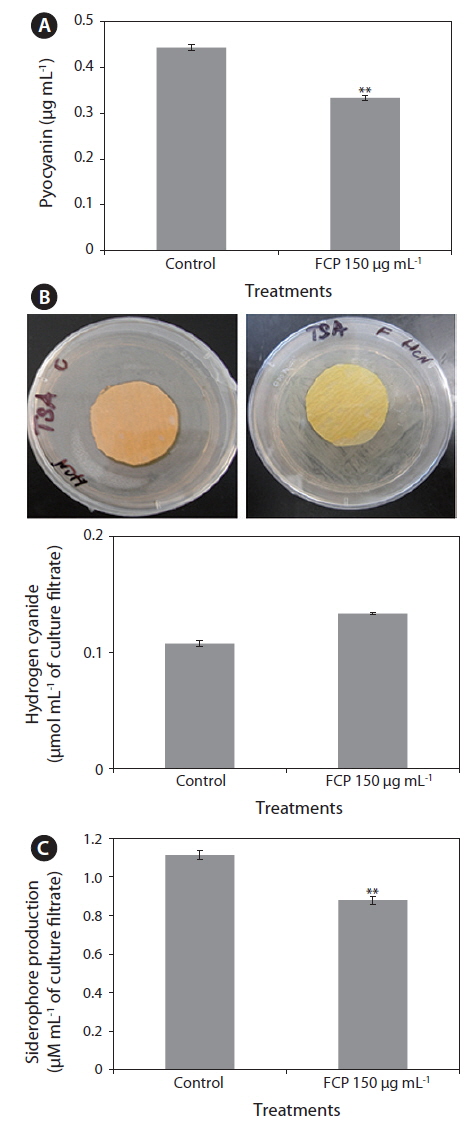
The highly diffusible, pigmented, phenazines, and two other (i.e., HCN and siderophores) toxic secondary metabolites, are known for their key roles in direct killing of infected organisms. The amount of pyocyanin present in the culture filtrate was much lower FCP-treated cultures (0.33 µg mL-1), as compared to the control (0.45 µg mL-1) (Fig. 2A). The HCN production of PA14 was reduced to 50% with FCP treatment (Fig. 2B). In addition, the FCP-treatment greatly reduced siderophore production (p ≤ 0.001) (Fig. 2C).
>
FCP inhibited Pseudomonas aeruginosa secretary virulence factors
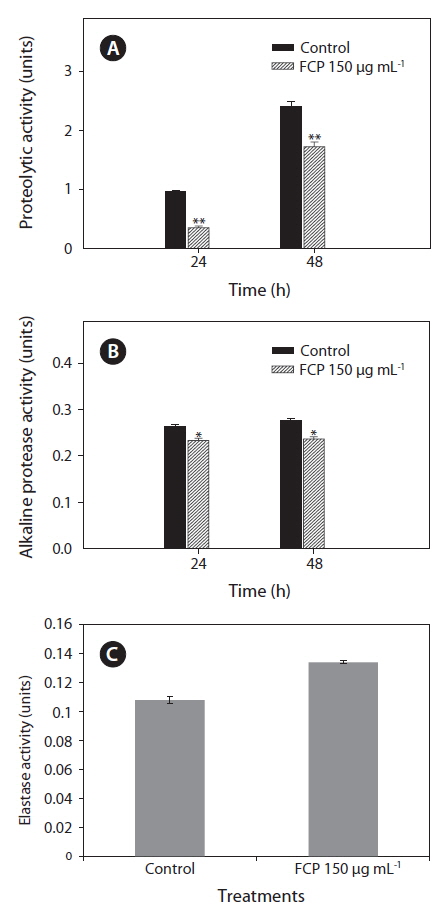
To further support the data, the effect of FCP on secretary virulence factors: i.e., protease, alkaline protease and elastase, of the pathogen PA14 were studied by supplementing bacterial growth medium with FCP. It was observed that proteolytic enzyme activity was reduced from 1.0 to 0.4 units (after 24 h) and 2.5 to 1.8 units (after 48 h) due to FCP-treatment (p < 0.001) (Fig. 3A). A similar trend in reduction was also noticed with alkaline protease activity (p < 0.01) (Fig. 3B). However, the elastase enzyme activity (p < 0.01) was found to be increased due to FCP treatment (Fig. 3C).
>
FCP suppressed the expression of Pseudomonas aeruginosa quorum sensing genes
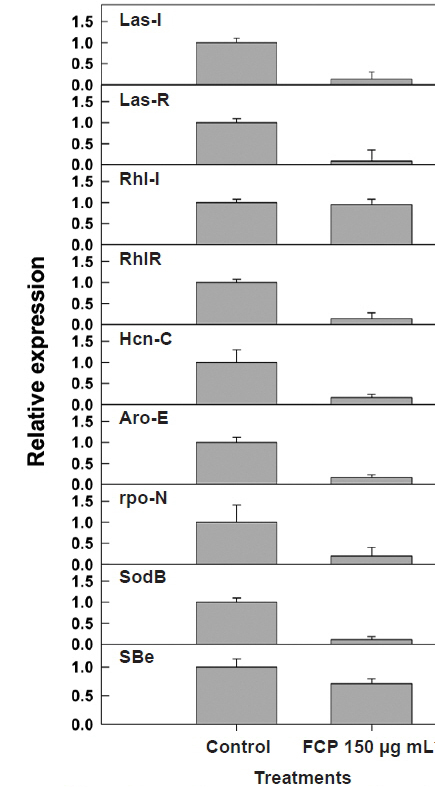
Both the
To validate the results from the biochemical analysis of secondary metabolite production, following FCPtreatment, gene expression studies were conducted using quantitative RT-PCR. The relative expression of major QS (i.e.,
>
FCP-treatment affected Pseudomonas aeruginosa motility
Since adherence, motility, biofilm formation, virulence and pathogenesis are associated and are involved in pathogenesis, we studied the effect of FCP-treatment on PA14 motility. FCP-treatments significantly affected swimming, swarming and the “twitching” motility of PA14 (Fig. 5). The direct effect of FCP-treatments on PA14 motility might, at least in part, contribute to reduced virulence on
>
FCP protects Caenorhabditis elegans from pathogen infection
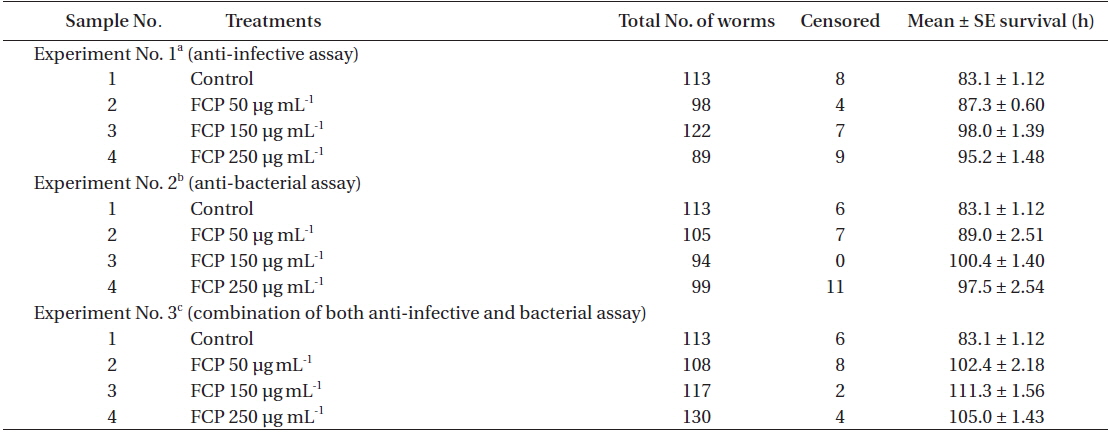
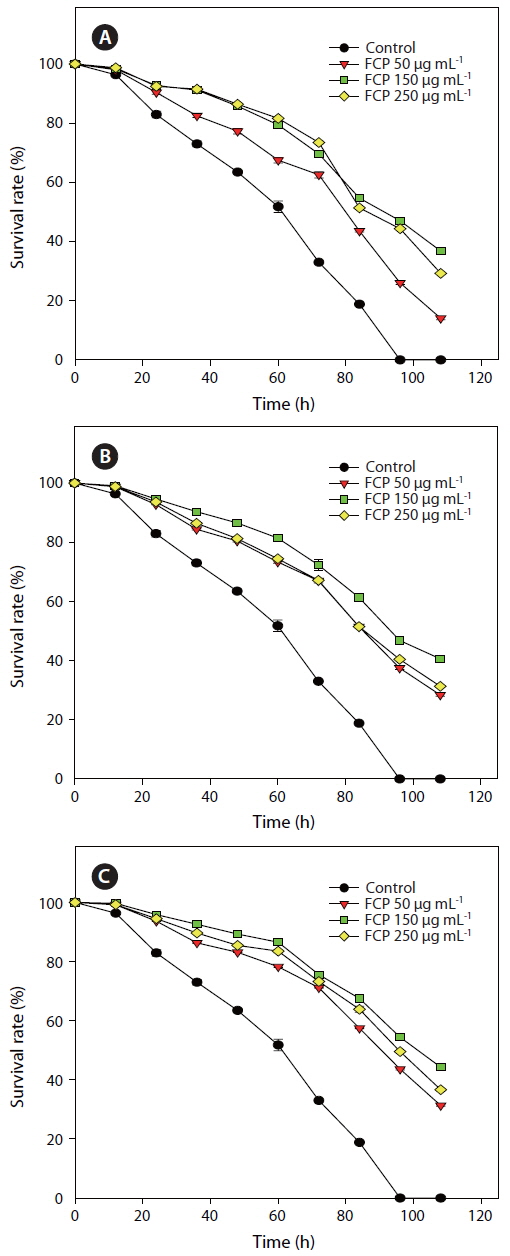
Three concentrations of FCP were tested to identify the role of the sulphated fucans in the
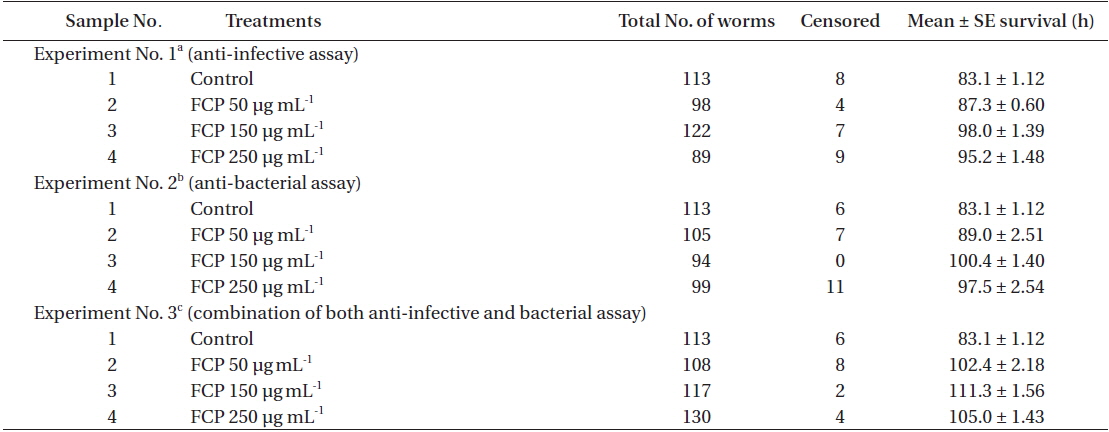
Effect of dietary treatment of FCP on the survival of Caenorhabditis elegans (wild type N2) challenged with Pseudomonas aeruginosa PA14
>
FCP alters Caenorhabditis elegans immune gene expression against pathogen infection
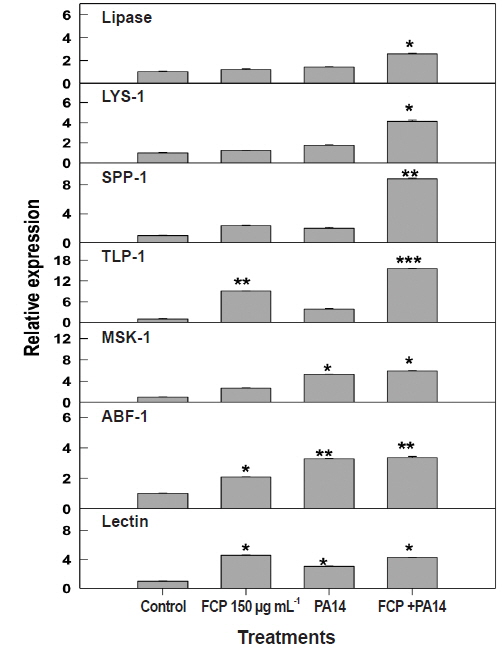
The expression of genes present in immune response pathways varies widely, according to host innate immunity. We chose to test the modulation in the level of expression of seven key genes, i.e., lipases, lysozyme, saponin-like protein, thaumatin-like protein, matridin SK domain protein, anti-bacterial protein, and lectin family protein from different immune response pathways (Appendix 3) based on FCP dietary treatment. FCP treatment caused a substantial up-regulation of all the genes tested. Expression of
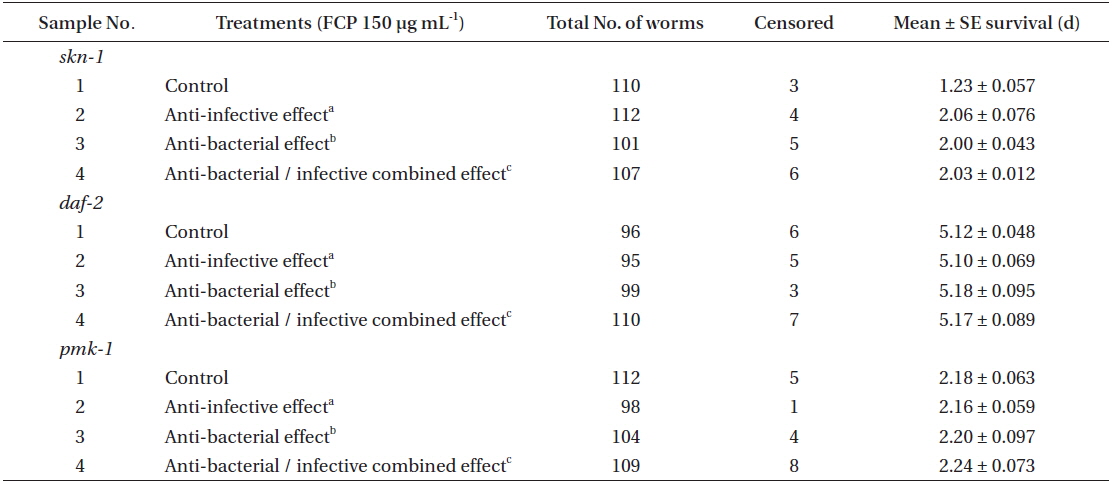
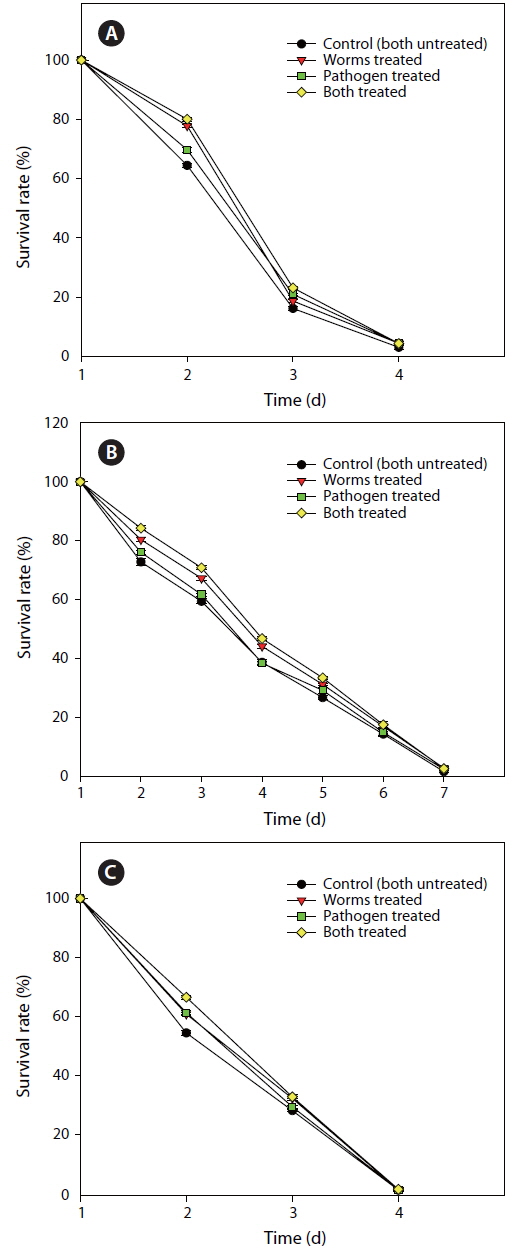
Effect of FCP on the survival of different functional mutant, of Caenorhabditis elegans challenged with Pseudomonas aeruginosa PA14
>
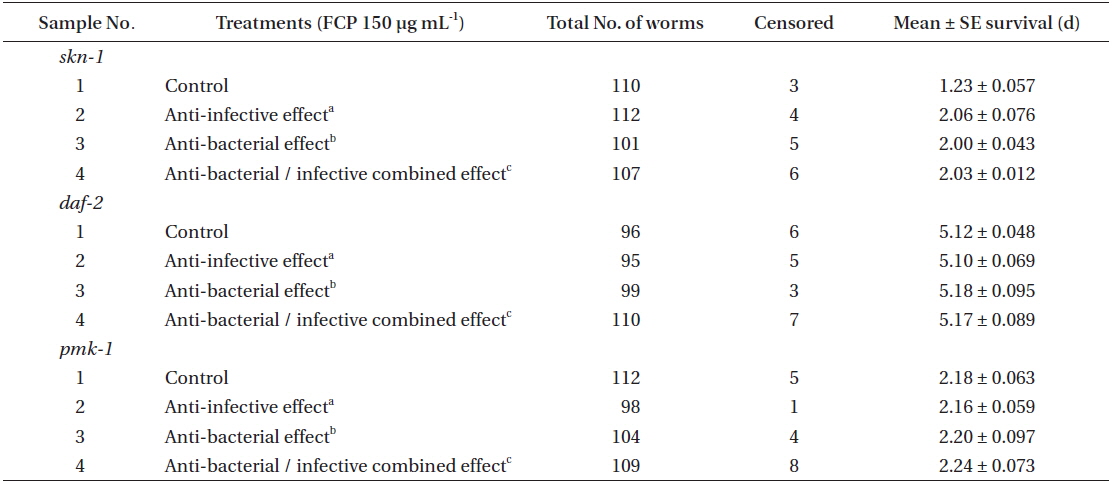
FCP protects worms against pathogen infection by altering functional pathways
From the previous experiment, it was inferred that FCP, at 150 µg mL-1, was the most effective in protecting to
In recent years the pharmaceutical industry have shown considerable interest in the biological activity of seaweed-derived sulphated polysaccharides (SPs) (Craigie 2011, Fitton 2011). Large proportions of SPs are L-fucose; sulphates along with small amount of other sugars such as xylose, galactose, mannose, etc. (Bilan et al. 2006). SPs have shown to possess anti-angiogenic, anti-tumor (Koyanagi et al. 2003), cell-mediated immune cell modulation (Cumashi et al. 2007), anti-metastatic fibrino-lytic (Alekseyenko et al. 2007) and anti-thrombotic properties (Cumashi et al. 2007). Anti-viral activities of fucoidan(s) from selected brown algae were well demonstrated by Hayashi et al. (2008) and Trinchero et al. (2009). Reilly et al. (2008) reported the the benefical effects of SPs in weaning pigs. SPs are effective free-radical scavengers that prevent oxidative damage and a wide range of age-related and degenerative conditions (Fujiki et al. 1992). It was recently reported that the FCP (Kandasamy et al. 2014) and water extract of Tasco® (a commercial product from
Conservedsignal transductionin
We can conclude that treatment with FCP, even at a low concentration, induced the innate immune system of