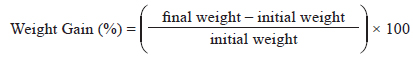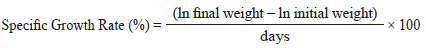Mercury (Hg) is a naturally occurring element found in air, water, and soil. It exists in the environment in three oxidation states Hg (0), Hg (I), and Hg (II). Microbial methylation in aquatic ecosystems is a crucial component of the Hg cycle in the environment (Wiener et al., 2003). Once in surface water, Hg enters a complex cycle whereby one form can be converted to another. Mercury attached to particles can settle onto sediments where it can diffuse into the water column, be resuspended, and be buried by other sediments, or be methylated. Methylmercury (MeHg) can enter the food chain, or it can be released back to the atmosphere by volatilization (During et al., 2009).
Fish naturally absorb MeHg into their tissues directly from water as it passes over their gills and by eating other contaminated foods, including fish (Roe, 2003). Mercury contamination in commercial fish feeds also occurs due to high metal levels in the raw materials (Wang et al., 2012). For this reason, dietary exposure is one of the routes of Hg contamination in fish (Choi and Cech, 1998). As a result, Hg has been regarded as undesirable substance in animal feed (EFSA, 2008).
Seafood contamination by MeHg is a public health concern, particularly in countries with high rates of fish consumption, such as Korea. In line with this, olive flounder is among marine fishes being preferred by consumers in Korea. Daily seafood consumption has reached 50.6 g, which accounts for ~3.8% of the total food ingested (Moon et al., 2009; Choi et al., 2012). Consequently, high blood Hg levels in a representative sample of the Korean adult population were associated with fish consumption (Kim and Lee, 2010). Accordingly, Moon et al. (2011) suggested implementation of systematic monitoring programs for seafood contamination by Hg in Korea. Limiting fish consumption was also suggested as a mechanism to avoid MeHg intake (Dorea and Barbosa, 2005).
Methylmercury is a neurotoxicant that affects the developing nervous system of humans and has been linked to neurological problems (Davidson et al., 2010). The various toxic effects induced by Hg in biological systems are due to alterations in the antioxidant defense system (Sheweita, 1998; Berntssen et al., 2003; Alves et al., 2007; Berg et al., 2010). Moreover, MeHg has been shown to cause coronary heart disease in humans (Wang et al., 2012) and decreased levels of nutrients in rats (Fukino et al., 1984).
Antioxidants such as vitamin C, vitamin E, and selenium (Se) decrease Hg toxicity in Japanese quail (Kung et al., 1987) and various other organisms (Chapman and Chan, 2000; Agarwal et al., 2010; Al-Attar, 2011). Bapu et al. (1994) examined the effects of vitamin C treatment after subcutaneous injections of methylmercuric chloride (MeHgCl) for 7 days in mice and found improved recovery of enzymatic activities.
Extensive work has emphasized the damage caused by heavy metal toxicity, but the combined ameliorative effect of vitamin C and E on MeHg contamination in fish should be investigated. Thus, the objective of this study was to evaluate the combined effects of vitamin C and E on tissue Hg, as well as growth-related problems, in juvenile olive flounder as the result of MeHg.
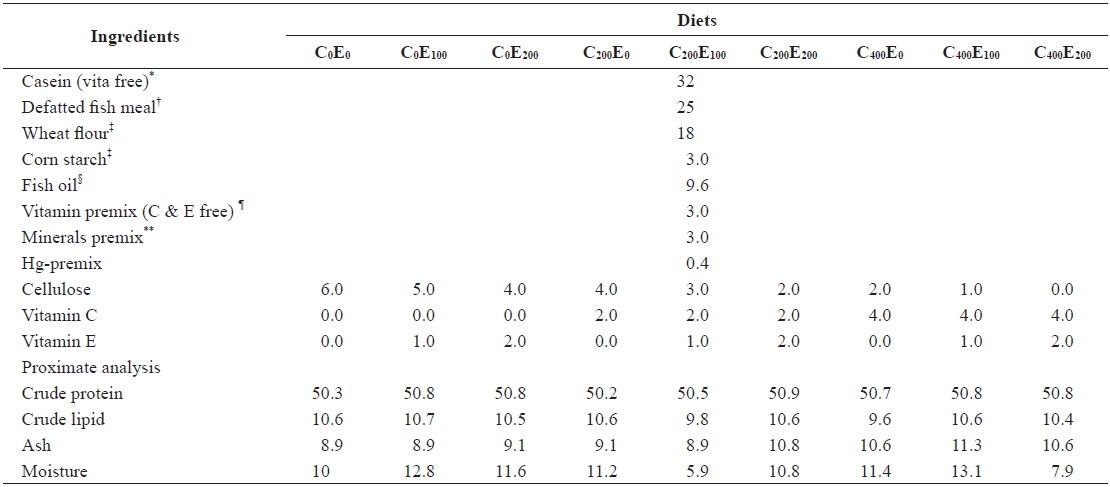
Nine diets with three vitamin C levels (0, 200 and 400 mg/kg diet in the form of l-ascorbyl-2-monophosphate) and three vitamin E levels (0, 100 and 200 mg/kg diet in the form of dl-α-tocopheryl acetate) with Hg (20 mg/kg Hg diet in the form of MeHg) were formulated (Tables 1 and 2). The 20 mg/kg MeHg diet was chosen based on a previous study (unpublished) that was conducted for 5 weeks in our laboratory and showed relatively lower mortality. In diets supplemented with MeHg and ascorbic acid sources, an equivalent amount of cellulose was removed. In a 3 × 3 factorial design these nine experimental diets (C0E0, C0E100, C0E200, C200E0, C200E100, C200E200, C400E0, C400E100 and C400E200) were formulated to be isonitrogenous and isoenergetic, containing 50% crude protein (CP) and 16.7 kJ available energy/g diet. The energy levels of diets were calculated based on 16.7, 16.7, and 37.7 kJ g-1 for protein, carbohydrate, and lipid, respectively (NRC, 2011). Vitamin-free casein was used as the main protein source. All the ingredients were mixed completely and then pelleted using 1- and 2-mm diameter dies (Bai and Lee, 1998). After processing, all diets were packed into small bags and stored at -20℃ until use.
[Table 1.] Composition of the experimental diets (% of dry matter basis)
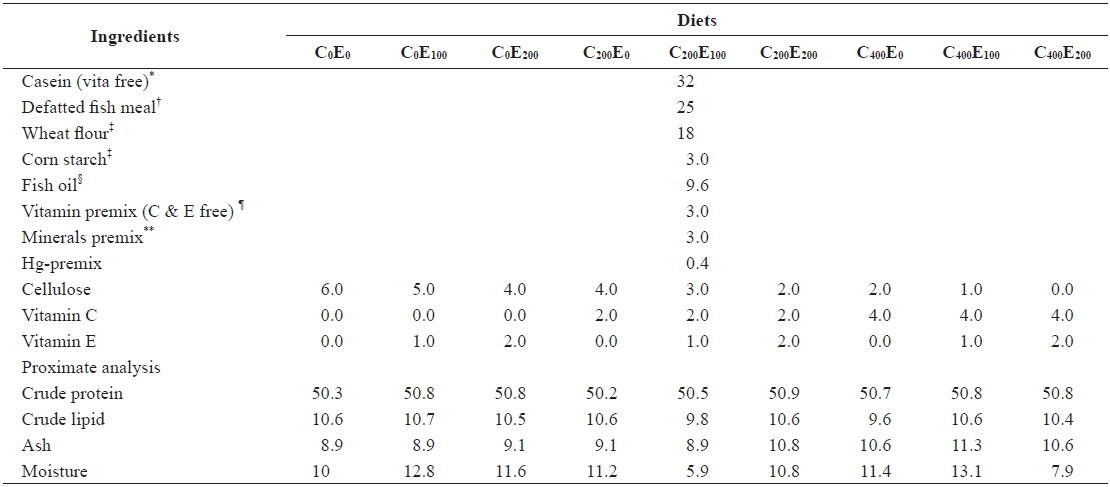
Composition of the experimental diets (% of dry matter basis)
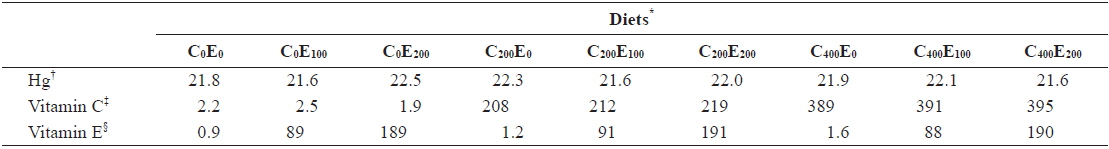
[Table 2.] Analyzed concentration of Hg, vitamin C and E of the experimental diets (mg/kg)
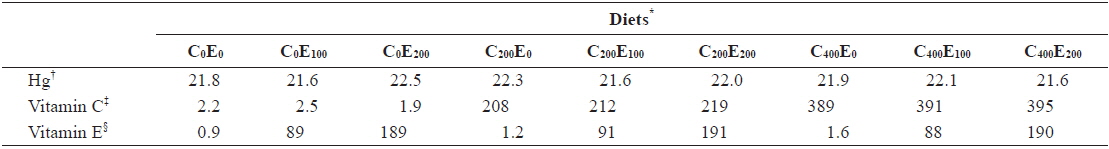
Analyzed concentration of Hg, vitamin C and E of the experimental diets (mg/kg)
>
Experimental fish and feeding trials
Juvenile olive flounder were obtained from Tong-Yeong, Korea. Prior to the start of the feeding trial, fish were fed the basal diet for 10 days as an adjustment to the semi-purified diet, and to deplete vitamin C reserves. The feeding trial was performed in a flow through system with 30 L aquaria receiving filtered seawater at a rate of 2 L/min. Supplemental aeration was provided to maintain dissolved oxygen near saturation. Water temperature was kept at 20 ± 1℃. Twenty experimental fish with a mean weight of 2.3 ± 0.05 g (mean ± SD) were randomly distributed into each aquarium. Each diet was fed to triplicate groups to satiation level three times per day at a feeding rate of 2.0 to 3.5% of wet body weight. Total fish weight in each aquarium was determined every 3 weeks, and the amount of diet fed to the fish was adjusted accordingly.
Growth performance was evaluated using weight gain (WG), specific growth rate (SGR), feed efficiency (FE), and the protein efficiency ratio (PER).
WG was calculated using the following formula:
SGR was calculated using the following formula:
FE and the PER were calculated using the following formulas:
>
Sample collection and analysis
After the final weighing, three fish were randomly removed from each aquarium and sacrificed with a lethal dose of benzocaine anesthetic (CAS, Canada). Proximate composition analyses of experimental diets were performed using standard methods (AOAC, 1995). Samples of diets were dried to a constant weight at 105℃ to determine moisture content. Ash content was determined by incineration at 550℃, crude lipid content was determined by Soxhlet extraction using the SOXTEC SYSTEM 1046 (FOSS, Hoganas, Sweden), and crude protein content was determined by the Kjeldahl method (N × 6.25), after acid digestion.
Ascorbic acid and α-tocopherol concentrations of the diet and tissue were determined by high performance liquid chromatography (HPLC; DIONEX, SOFTRON, USA). For ascorbic acid, the ultraviolet detector was set to 254 nm. The mobile phases for ascorbic acid and α-tocopherol were 0.05 M KH2PO4 and hexane: isopropanol (98:2, v/v), respectively. The flow rate for both was 1.0 mL/min. Weighed samples were homogenized in 10% cold metaphosphoric acid (for ascorbic acid) and in 5-mL ethanol (for α-tocopherol). Homogenates were centrifuged at 3,000
Greater than 90% of Hg present in fish is in the form of MeHg. Therefore, the total concentration of Hg was measured instead of MeHg (Bloom, 1992; Amlund et al., 2007). A direct Hg analyzer (DMA-80, Milestone, Inc., Shelton, CT) was used to determine the tissue Hg concentration; the method followed that of Lee et al. (2011). A certified reference material (DORM-2 dogfish liver, National Research Council, Canada) was used simultaneously during the analyses.
Data were analyzed by two-way ANOVA to test the effect of the dietary treatments. Least significant difference (LSD) was used to compare means when a significant treatment effect was identified. SPSS version 16.0 (SPSS Inc., Chicago, IL, USA) was used and
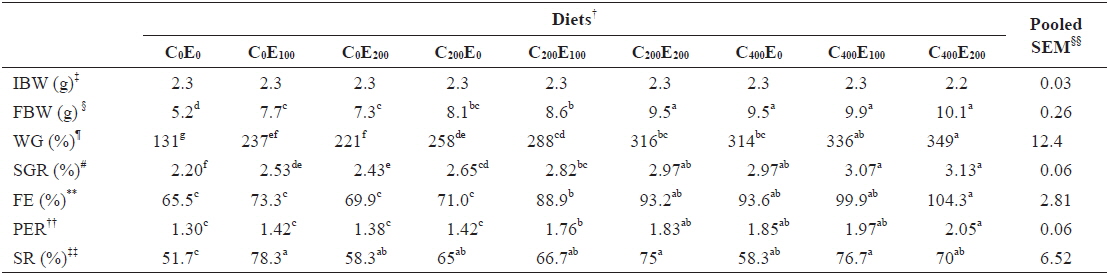
After 8 weeks of feeding, fish that were fed 400 mg/kg vitamin C at all vitamin E levels had better growth performance, compared to the other groups. Significantly higher (
[Table 3.] Growth performance of juvenile olive flounder fed experimental diet for 8 weeks*
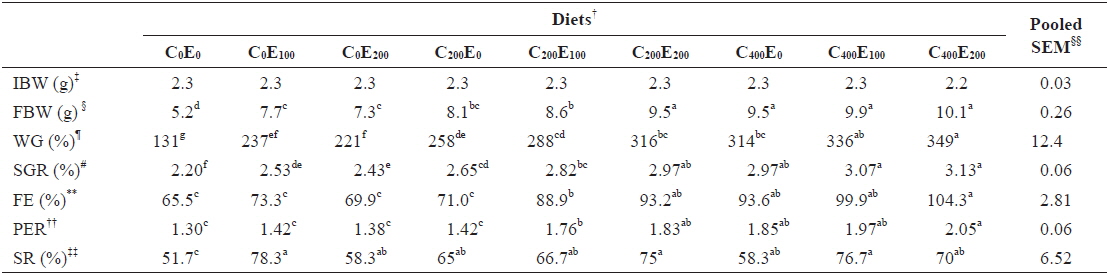
Growth performance of juvenile olive flounder fed experimental diet for 8 weeks*
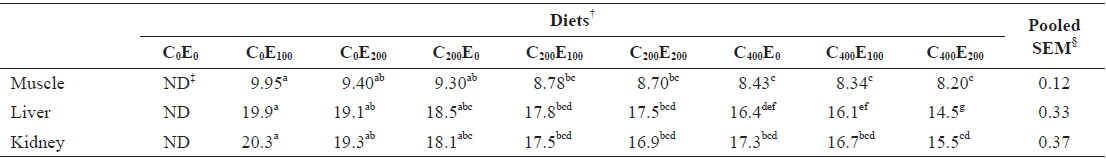
Muscle Hg concentrations in fish that were fed diets comprising 200 and 400 mg/kg vitamin C were significantly lower (
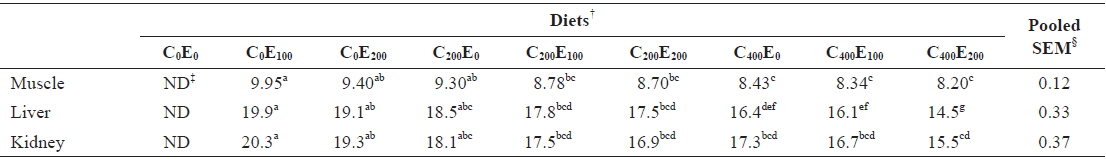
Mercury concentrations (μg/g of wet matter basis) in muscle, liver and kidney of juvenile olive flounder fed the experimental diets for 8 weeks*
Although the effects of Hg on fish are complex and multiple (Weis, 2009), MeHg is known to cause oxidative stress, causing damage to vital components of the biological system, from mitochondrial damage to altered behavior (Berntssen et al., 2003; Alves et al., 2007; Berg et al., 2010). The high affinity of methylmercury for the thiol or sulfhydryl (-SH) groups of proteins underlies its mechanisms of toxicity (NRC, 2005). Therefore, the poor growth performance observed in olive flounder fed diets that did not contain vitamin C and/or E (C0E0, C0E100, C0E200, C200E0 and C400E0) might have occurred due to decreased enzyme activity, altered structural functionality, or transport process problems caused by Hg accumulation (Zalups and Lash, 1994). All such functional disturbances might have been caused by excessive release of reactive oxygen species (ROS) driven by Hg accumulation (Bansal et al., 1992; Lund et al., 1993). Consistent with the findings in this study, impaired growth and poor gonad development caused by dietary MeHg have been reported in juvenile walleye (Friedmann et al., 1996), Sacramento blackfish (Houck and Cech Jr, 2004) and Green & White sturgeon (Lee et al., 2011).
Excessive release of ROS and increased lipid peroxidation in cells are harmful effects that occur during Hg accumulation (Bansal et al., 1992; Lund et al., 1993). Free radicals and the products of peroxidation damage the integrity and function of biomembranes. Vitamin C and E inhibit free radical formation and lipid peroxidation (Sies et al., 1992; Aldana et al., 2001; Rao and Sharma, 2001; Kalender et al., 2004; Valko et al., 2005; Agarwal et al., 2010). Low Hg concentrations were observed in muscle and liver of fish that were fed the C400E200 and C400E100, and C400E200 diets, respectively. This was presumably due to the ability of the antioxidant vitamins to neutralize mercury ions, or bind with transition metals and prevent ROS-mediated oxidative damage in tissues (Ganther, 1980; Nandini and Lata, 2010).
In the present study, vitamins C and E acted synergistically against Hg accumulation, possibly because of the tendency of vitamin E to maintain vitamin C levels in damaged tissues by inhibiting free radical formation (Duval and Poelman, 1995). Vitamin C is also known to regenerate vitamin E from its oxidized form (Bruno et al., 2006). Therefore, effective dispersal of Hg from –SH groups by these vitamins, along with their ability to inhibit and remove free radicals, might have reduced tissue Hg concentrations and helped to improve growth performance (Fukino et al., 1984; Patil and Rao, 1999; Durak et al., 2010). In agreement with our findings, Guillot et al. (1998) reported that administration of 1,000 mg/kg ascorbic acid reduced Hg accumulation in multiple rat tissues. Rambeck et al. (1996) also confirmed the role of ascorbic acid to reduce the retention of inhaled Hg vapor. Durak et al. (2010) strengthened the present study`s findings by showing amelioration of Hg-induced toxicity through the combined effects of vitamin C and E,
In conclusion, poor growth performance resulting from MeHg accumulation were alleviated and the Hg concentration in muscle, liver, and kidney were reduced by dietary supplementation of vitamins C (200 and 400 mg/kg) and E (100 and 200 mg/kg) in juvenile olive flounder.