The Caspian roach,
In order to improve production efficiency in hatcheries, it is important to optimize the conditions in which the fish are reared. These conditions can include the physical culture environment (temperature, salinity, light intensity and photoperiod) and general nutritional parameters such as diet composition, ration and feeding frequency (Mohseni et al., 2006). The strategy of their rearing condition will help us to gain best results in their culture and can affect their growth and survival. However, there is no information on the effect of photoperiod manipulation on the growth performance of juvenile Caspian roach. Therefore, the aim of the present study was to investigate the effect of photoperiod manipulation on growth performance and hematological parameters of juvenile Caspian roach,
Juvenile Caspian roach,
>
Sampling and parameters estimation
At the termination of the feeding trial, fish were starved for 24 h and the total number and weight of fish in each tank was determined for calculation of weight gain (WG), specific growth rate (SGR), feed efficiency (FE), feed intake (FI) and survival. After obtaining the final total weight, five fish were randomly selected from each tank and blood samples were withdrawn from the caudal vein using a 26-gauge hypodermic needle on a 1-ml syringe and transferred to tubes that were kept on ice until centrifugation at 1,600 (g) for 10 min. Hematocrit (PCV) was determined using the microhematocrit method (Brown, 1980) and hemoglobin (Hb) was measured by the cyanmethemoglobin procedure using Drabkin’s solution. Hb standard prepared from human blood (Sigma Chemical Co., St Louis, MO, USA) was used. Plasma glucose was measured by the enzymatic glucose-HK procedure (Sigma). We also calculated number of white blood cells (WBCs), and number of red blood cells (RBCs) according to Ranzani-Paiva et al. (2004). The rest of the fish were freeze-dried as whole body and held at −80℃ until used for proximate composition analysis. The samples of diet and whole-fish body from each treatment were analyzed according to the standard methods of AOAC (2000) for moisture, protein, lipid, and ash. Moisture content of samples was estimated by drying oven at 135℃ for 2 h to constant weight. Crude protein was determined using the Kjeldahl method (N × 6.25) after acid digestion. Crude lipid was determined by soxhlet extraction using the Soxtec system 1046 (Tacator AB, Hoganas, Sweden), and ash was determined by combusting dry samples in a muffle furnace at 550 ℃ for 6 h.
Data were subjected to one-way analysis of variance test using Statistix 3.1 (Analytical Software, St Paul, MN, USA). When a significant treatment effect was observed, a Least Significant Difference test was used to compare means. Treatment effects were considered significant at
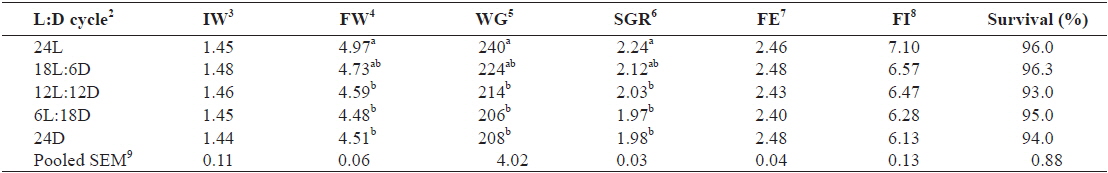
Table 1 shows the growth performance parameters of juvenile Caspian roach exposed to different photoperiod regimes. At the end of 8 weeks of experiment, FW, WG and SGR of fish exposed to 24L photoperiod were significantly higher than those of fish exposed under 12L:12D, 6L:18D and 24D photoperiods (
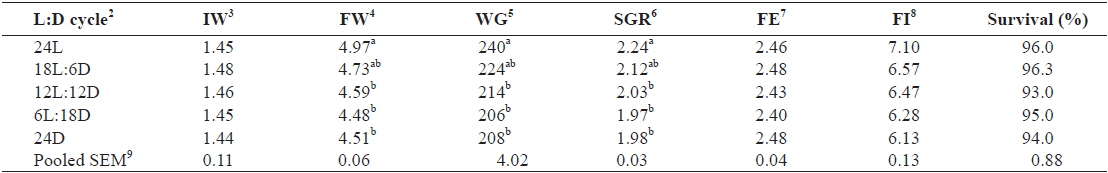
Effect of different photoperiods on growth performance of juvenile Caspian roach Rutilus rutilus caspicus for 8 weeks1
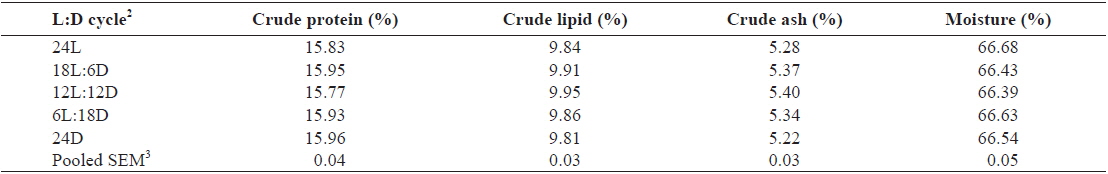
Whole-body proximate composition of juvenile Caspian roach exposed to different photoperiod regimes are shown in Table 2. There were no significant differences in whole-body protein, lipid, moisture and ash contents of fish among all treatments (
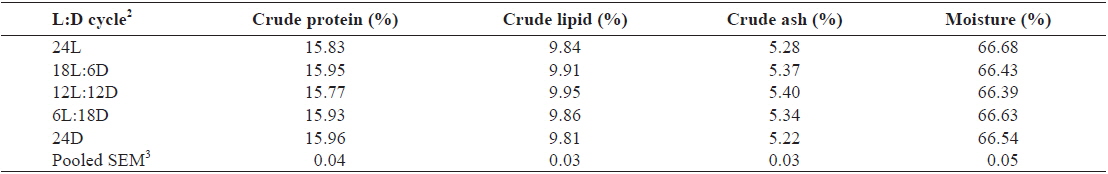
Effect of different photoperiods on whole body proximate composition (% of DM basis) of juvenile Caspian roach Rutilus rutilus caspicus for 8 weeks1
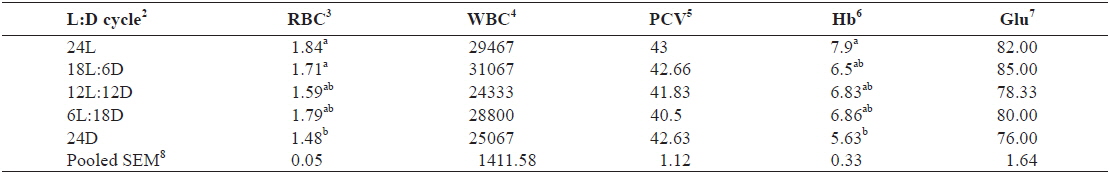
The hematological parameters of juvenile Caspian roach exposed to different photoperiod regimes are shown in Table 3. RBC of fish reared under 24L and 18L:6D photoperiods was significantly higher than those of fish exposed under 24D photoperiod (
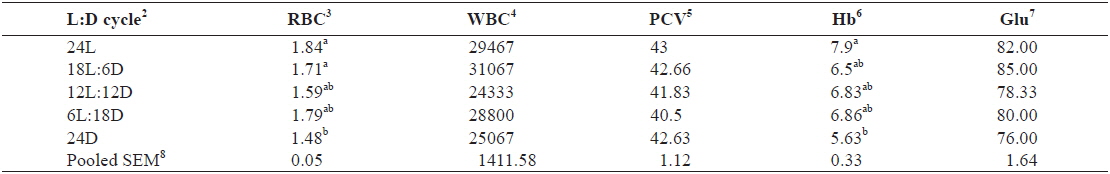
Effect of different photoperiods on hematological parameters of juvenile Caspian roach Rutilus rutilus caspicus for 8 weeks1
During the 8 weeks of rearing, survival ranged between 93% and 96% and averaged 94.9%. The results of the present study indicated that juvenile Caspian roach were exposed to 24L and 18L:6D photoperiods had significantly better growth, WG and SGR than those exposed to 12L:12D, 6L:18D and 24D photoperiods. Similar results have been reported that growth performance could be enhanced by continuous light or extending the light photoperiod in Atlantic salmon juvenile,
Photoperiod is commonly believed to be one of the directive factors that could control fish growth during their early life stage through its influence on endogenous feeding rhythms and efficiency or food availability (Björnsson, 1997; Boeuf and Le Bail, 1999; Taylor et al., 2006). The finding that higher growth accompanied with both higher feed intake and feed efficiency under long and continuous photoperiods parallels the findings in other species, such as haddock,
It is well known that hematological parameters regarded as reliable indicators for assessment of health status in fish, but they can vary with season, temperature and nutritional status (Bond, 1979). In this study, the results demonstrated that photoperiod manipulation cause significant differences in the levels of hematological parameters such as RBC and Hb; but, did not cause significant in the PCV and WBC. The PCV has been shown to increase under stressful conditions (Pierson et al., 2004) and this could be attributed to the swelling of red blood cells (Biswas et al., 2006). The PCV has also been shown to decrease under chronic stress (Barcellos et al., 2004).
In addition, there was no significant difference in plasma glucose among the treatments in this study. Plasma glucose concentrations have long been used as indicators of stress in fish (Hattingh, 1976; Donaldson, 1981; Wedemeyer and McLeay, 1981). Yet, in many studies (Adams et al., 1985; Brown et al., 1986; Goss and Wood, 1988; Pottinger et al., 2002), under stress plasma glucose either remained unchanged or took a longer duration of stress to show the change. The results demonstrated that photoperiod manipulation did not cause a significant acute stress response in juvenile caspian roach as the levels of different stress indicators in fish exposed to different photoperiods. It has been demonstrated that chronic stress generally results in a higher elevation of cortisol and glucose concentrations in fish (Leonardi and Klempau, 2003). In this study, plasma cortisol was not determined; however, plasma glucose concentration in the blood indicates that photoperiod manipulation did not appear to cause significant chronic stress response in the fish. Similarly, the findings for juvenile red sea bream,
In conclusion, the results suggested that the growth performance of juvenile caspian roach can be stimulated remarkably by the manipulated photoperiods used in 24L and 18L:6D photoperiods. The higher growth performance under manipulated photoperiods may be attributed to the feeding time as well as to improved appetite, greater feed intake and higher feed conversion efficiency. In addition, the photoperiod manipulation used in this study did not cause a significant stress response, and can therefore be used to stimulate the growth performance of juvenile Caspian roach,