Among diverse acupuncture treatments in Korea, pharmacopuncture is a new and unique treatment method that combines acupuncture based on the meridian theory and herbal medicine based on Qi and flavor theory [1]. Meridian field pharmacopuncture (MFP) is a representative pharmacopuncture method. MFP is divided into aromatic and oil-based MFP. Oil-based pharmacopuncture is administrated to disorders caused by deficient body lubrication, and
The safety of injecting herbal medicine into a muscle or a vein is very important. Water-soluble
This study was approved by Institutional Animal Care and Use Committee of Biotoxtech Co. (Approval No: 130365). The tests were performed according to Good Laboratory Practice (GLP) regulation and toxicity test guidelines of the Ministry of Food and Drug Safety (MFDS).
To process the tissues of deer antlers (DA), we chopped 35 g of DA into small pieces and put them into 500 mL of 95% ethanol. The DA mixture was stirred 4 hours a day during cold storage for one week to extract all DA components; then, the stirred DA mixture was filtered with a Whatman™ nylon membrane filter (pore size: 0.45 μm), and 12 g of the filtered mixture was added to 1000 cc of safflower oil in a round flask. This was followed by decompressed concentration to volatilize ethanol completely. Ethanol vaporized at 55°C and was liquefied through the cooling tube; the DA components were dissolved into the safflower oil. The liquid ethanol was discarded, and the DA extract solution was transferred to the preparation tank. The transferred DA and safflower oil solution (CFC solution) was mixed by using nitrogen gas (N2, 99.99% purity) agitation for over an hour. The CFC solution was filtered with 0.2 μm pore filter paper (KA2DFLP02, Pall Corporation, U.S.A.) to remove endotoxin, and the filtered solution was added to water for injection (WFI) at an adequate rate in the reaction container. The CFC solution and an emulsifier were mixed with high speed agitator to make a WCFC solution. The pH of the WCFC solution was adjusted to pH 7.3 by using NaOH. The WCFC solution was passed through a filter (0.1 μm Sartorius) between the preparation and the filling tanks and was transferred to the filling tank by using N2 gas. The WCFC solution was subdivided, placed into vials, and sterilized at high pressure (high pressure sterilizer, fine FA, Korea) at 121°C for 30 minutes. The vials containing the WCFC solution were kept refrigerated at 4°C.
Five-week-old male (n = 24) and female (n = 24) Sprague-Dawley (SD) rats with weights ranging from 112.2 to 125.6 g in males and from 110.5 ─ 131.0 g in females were purchased from Orientbio Inc., Korea, for use in this study. When the animals were received, they underwent basic visual examinations, and their weights were measured by using an electronic scale (CP3202S, Sartorius, Germany). General symptoms were observed daily during the 7-day stabilization period. On the last day of the stabilization period, weights, general symptoms and weight changes were measured to confirm the normal conditions for all animals.
Animals were kept in stainless-steel wire cages with dimensions of 260 W × 350 D × 210 H (mm) under the following conditions: temperature in the range of 22.0 ─ 23.9°C, relative humidity in the range of 50.3% ─ 77.3%, a air change rate of 10 ─ 15 times/hour, a illumination time of 12 hours/day (7:00 am ─ 7:00 pm), and an intensity of illumination of 150 ─ 300 Lux. The breeding cage was changed once every two weeks. The breeding apparatuses were cleaned by using an automatic washer and were sterilized by using an autoclave. Solid feed (Teklad Certified Irradiated Global 18% Protein Rodent Diet 2918C, Harlan Laboratories, Inc., U.S.A.) was put into the feeding system for free intake. Drinking water was sterilized by using an ultraviolet water sterilizer.
All animals were put into grouped on the last day of stabilization (grouping day). Twenty male and twenty female rats whose weights were approximately equal to the mean weight were selected. Selected animals were randomized into 4 groups with 5 male rats and 5 female rats for each group (male weights: 179.8 ─ 197.0 g, female weights: 145.7 ─ 177.0 g) so that average weights per group were about the same. The remaining animals were excluded from the study.
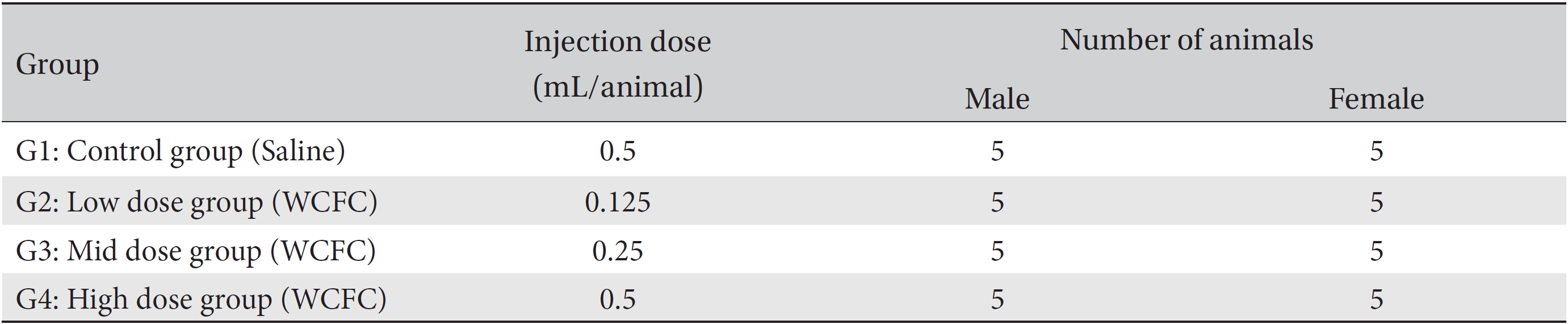
An intramuscular injection route using a method applicable to Korean medicine clinics was selected because many kinds of pharmacopunctures have been injected into muscle or subcutaneous tissue to treat diseases at Korean medicine clinics. Dosages for the control and the high dose groups were 0.5 mL/animal of saline and of WCFC, respectively, and those for the middle dose group and the low dose group were 0.25 and 0.125 mL/animal, respectively. WCFC was injected into the muscle of the left femoral region by using a disposable syringe (1 mL, 26 gauge) (Table 1).
In a pilot test, no deaths were observed for intramuscular injections of 0.5 mL/animal of WCFC into male and female rats (Biotoxtech Study No.: B13477P). Based on the pilot study and clinical experience, the injection doses selected for this study were 0.5 mL/animal for the high dose, 0.25 mL/animal for the middle dose, and 0.125 mL/animal for the low dose, the dose being reduced by a factor of 2 from group to group. Normal saline (Choongwae Pharma Corp., Korea) at a dose of 0.5 mL/animal was injected into the control group.
General clinical sign, such as types of toxicity, onset time, recovery time, etc., and deaths were observed 30 minutes, 1, 2, 4, and 6 hours after the first injection on the day of the first injection (day 0). General symptoms were observed daily for 14 days after injection. The body weights of the SD rats were measured on the day of the injection (before injection) and on the third, seventh, and fourteenth after the injection. Before necropsy, all animals were fasted for more than 18 hours. On the necropsy day (fifteenth day after injection), animals were anesthetized with isoflurane, and blood was taken from the abdominal aorta. Blood was kept in a 1-mL ethylenediaminetetraacetic acid (EDTA) tube, and hematologic tests were done by using a blood corpuscle analyzer (ADVIA 2120i, Siemens, Germany). The red blood cell count (RBC), hemoglobin concentration (HGB), hematocrits (HCT), mean corpuscular cell volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular cell hemoglobin concentration (MCHC), platelet count (PLT), white blood cell count (WBC), WBC differential counts (neutrophils, lymphocytes, monocytes, eosinophils, and basophils), and reticulocyte (Reti) count were measured. For blood coagulation tests, blood was drawn in 2-mL tubes that contained 3.2% sodium citrate. The blood was centrifuged at 3,000 rpm for 10 minutes to collect the plasma. The prothrombin time (PT) and the active partial thromboplastin time (APTT) were measured with a coagulation time analyzer (Coapresta 2000, Sekisui, Japan). The blood that remained after the hematologic test was used for the serum biochemical analysis. The blood was centrifuged at 3,000 rpm for 10 minutes to collect the serum. Blood urea nitrogen (BUN), creatinine (Cr), aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), gamma glutamyl transpeptidase (GGT), total protein (TP), albumin (Alb) and albumin/ globulin ratio (A/G ratio), total bilirubin (T-bili), total cholesterol (T-Chol), triglycerides (TGs), phosphorus (P), glucose (Glu), and calcium (Ca) were measured with a blood chemical test analyzer (7180, Hitachi, Japan). Sodium (Na+), potassium (K+) and chloride (Cl-) were measured with an electrolyte analyzer (AVL9181, Roche, Germany).
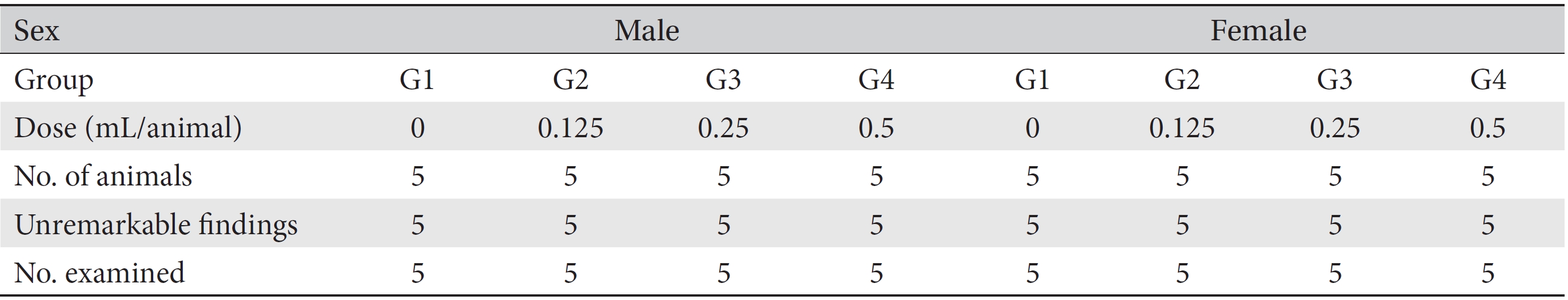
Detailed visual examinations of entire organs were done for all animals after necropsy had been conducted. From the necropsied animals, injection sites were removed and fixed with 10% neutral buffered formalin (NBF). Fixed tissues underwent a general tissue processing process such as dehydration, paraffin embedding, and sectioning to make tissue slices, which was followed by hematoxylin and eosin (H&E) staining. Residual tissues were preserved with 10% NBF. Microscopic examinations were conducted for all injection sites on all test group rats, and histopathologic examinations were performed for all injection sites of the rats in all groups.
The data for weights, hematology and serum biochemistry were analyzed by using a statistical analysis system (SAS, version 9.3, SAS Institute Inc., U.S.A.). The continuous data are presented as means ± standard deviations. The test for equality of variance was done with the Bartlett test (
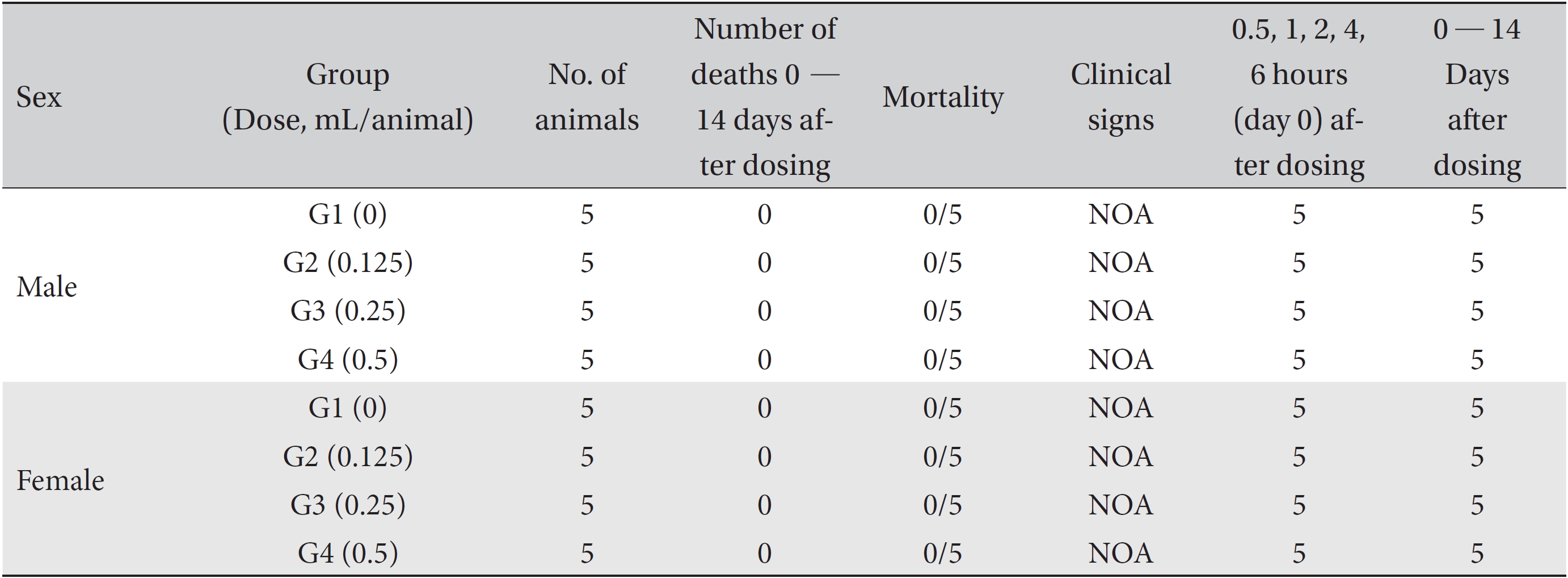
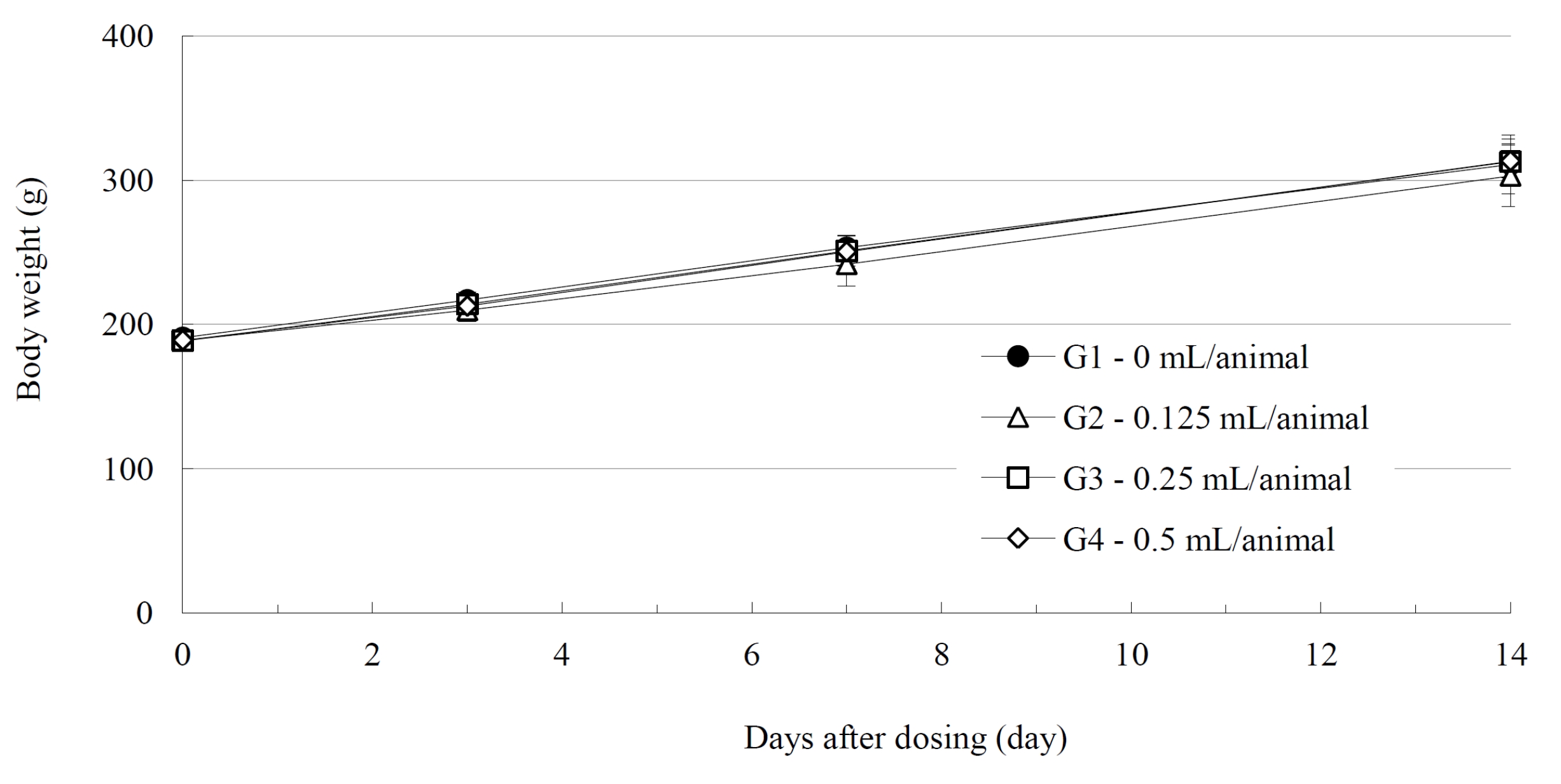
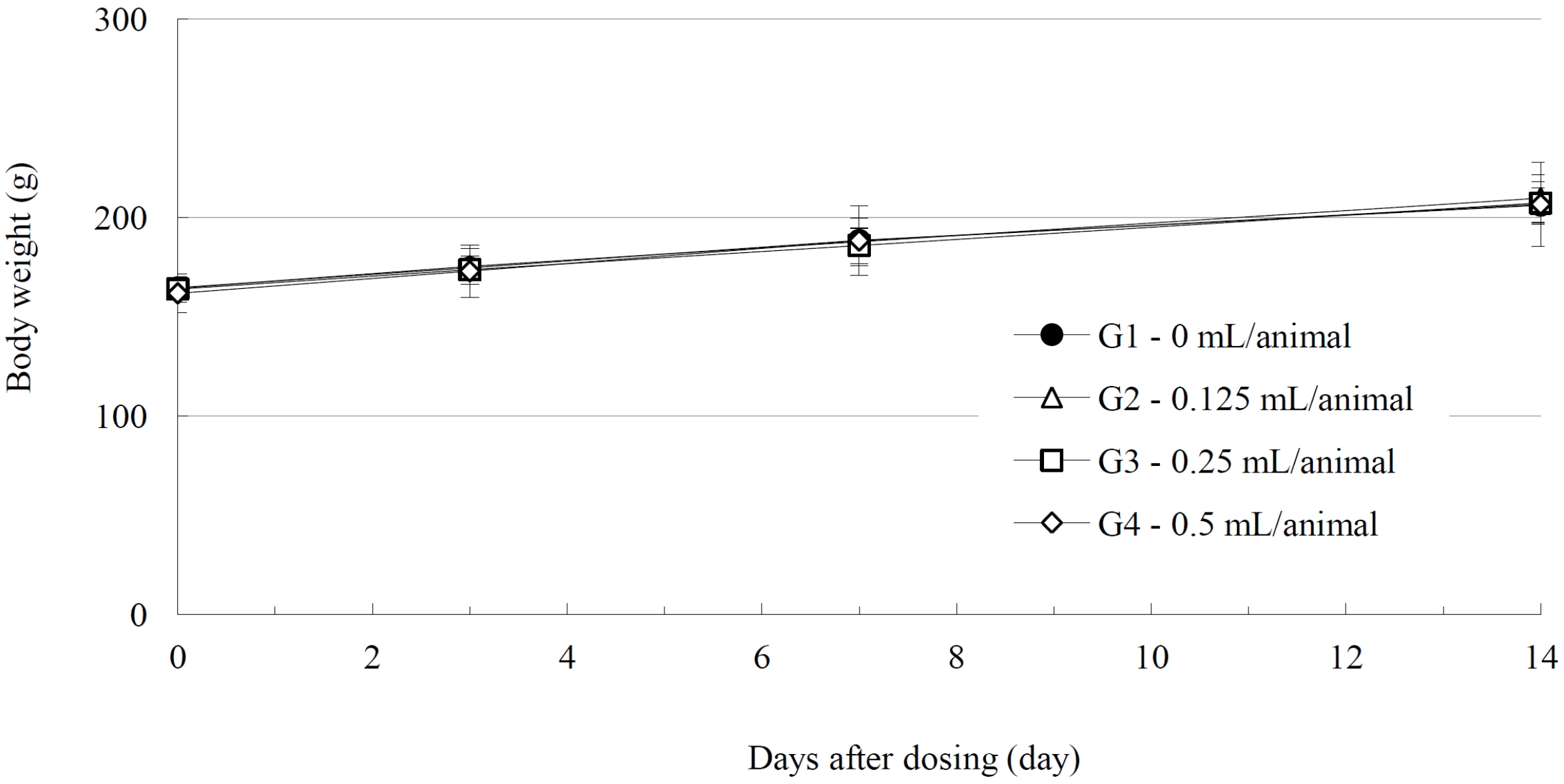
No deaths of either male or female SD rats were observed in either the control or the test (0.125, 0.25, and 0.5 mL/ animal) groups during the observation period (Table 2). No abnormal clinical symptoms were observed in either male or female rats in either the control or test the groups during the observation period (Table 2). No significant weight changes were noted for male and female SD rats in the test groups compared with those in the control group (Figs. 1,2).
Compared with control group, the test groups showed no significant differences in the hematological parameters, such as the RBC, HGB, HCT, MCV, MCH, MCHC, PLT, WBC, WBC differential count, and Reti count. No changes of parameters in serum biochemistry were observed in test group of male and female rats. No toxicological importance was found because the slight changes in the AST level in the female rat in the low dosage (0.125 mL/animal) group were sporadically observed with no dose dependence (AST in female group: 96.2 ± 23.7 U/L in the low dose (0.125 mL/animal) group
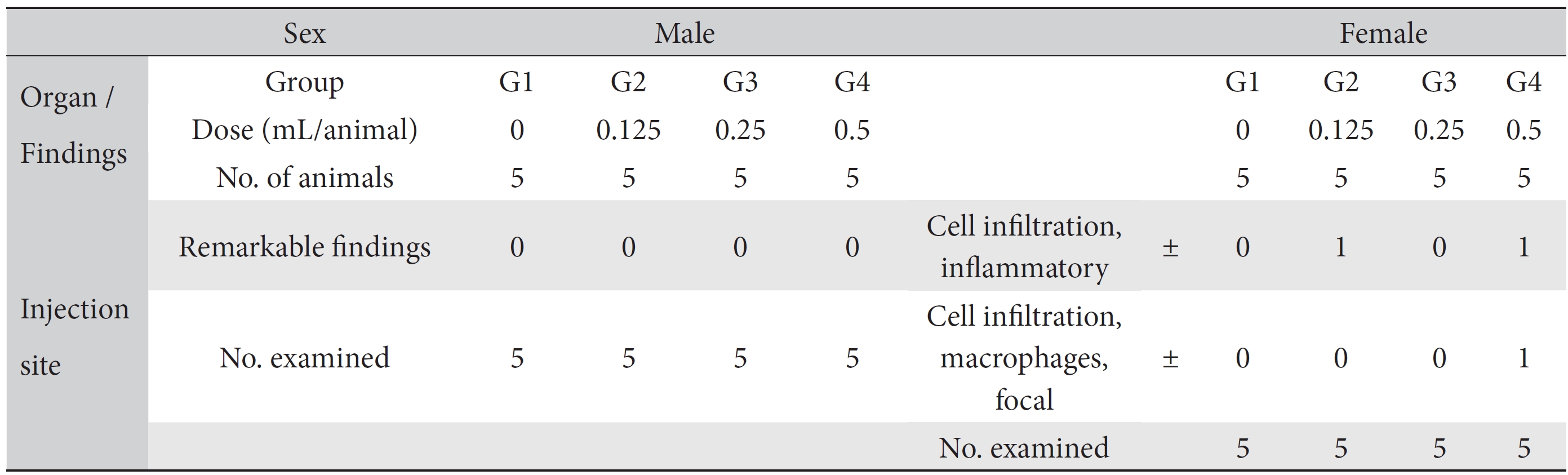
On the topical tolerance tests, no abnormal responses were noted at the injection sites on male and female SD rats in the test groups. In addition, histopathological tests showed that the observed changes, such as inflammatory cell infiltration in the low dose (0.125 mL/animal) group and inflammatory and focal macrophage cell infiltration in the high dose (0.5 mL/animal) group, appeared spontaneously and sporadically and showed no dose dependence (Table 4).
Meridian fields (MFs) are areas of the body’s functions that display similar properties for local disorders and are classified as wind, heat, fire, dry, damp, and cold MFs. The Heat MF is in wind, heat, dry, and fire MFs, and the Cold MF is in cold and damp MFs. The meridian body (MB) consists of the acupuncture points that have direct relevancy to a disease or disharmony, and it becomes sensitive and solidifies if the body is in a pathological state. The MB generates Qi and consumes lubrication (潤) to overcome the disorder. Body lubrication consists of nutrients, and Qi is energy. Both are used in the prevention and the treatment of the decrepitude and weak constitution. Diseases can be diagnosed and treated by using the principles of MFs and MB [1]. Aromatic and oil-based pharmacopuncture are two types of MFP that are applied to the MB or responsive points related to diseases. Aromatic pharmacopuncture is used to treat acute pain disorders caused by Qi stagnation and Blood stasis whereas oil-based pharmacopuncture is administrated to treat kinetic disorders caused by a deficiency of body lubrication [1].
CF (safflower) is the dried flowers of
CC is extracted from DA, which is called Pilose Antler or Pilose Deer-horn (common name) and
No mortalities or abnormal findings in body weights, clinical symptoms, and hematology and serum chemistry data were observed in this single-dose intramuscular-injection toxicology test. The histopathological test showed abnormal changes, such as inflammatory cell infiltration in the low dose (0.125 mL/animal) group and inflammatory and focal macrophage cell infiltration in the high dose (0.5 mL/animal) group (Table 4). However, we speculate that those abnormalities have no toxicological meaning because they were spontaneous and sporadic and showed no dose dependence. Therefore, based on the results of this toxicity study, we conclude that WCFC is safe. However, additional safety studies or toxicological tests, such as 4-week, repeated toxicity tests and so on, as well as efficacy studies, are needed.
The results of this single-dose toxicity study show that WCFC is safe because the lethal doses of WCFC in male and female SD rats were estimated to be higher than 0.5 mL/animal.
[Table. 1] Grouping of animals
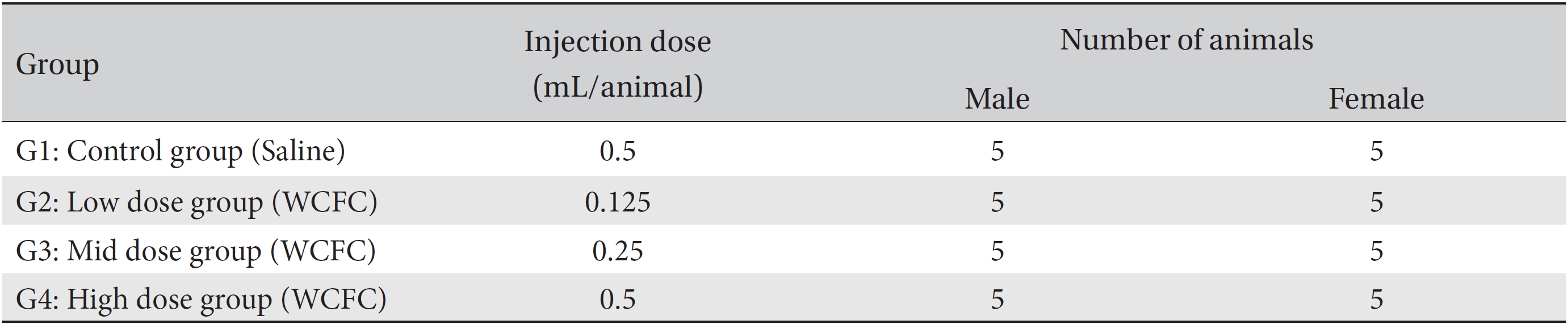
Grouping of animals
[Table. 2] Summary of mortality and clinical signs
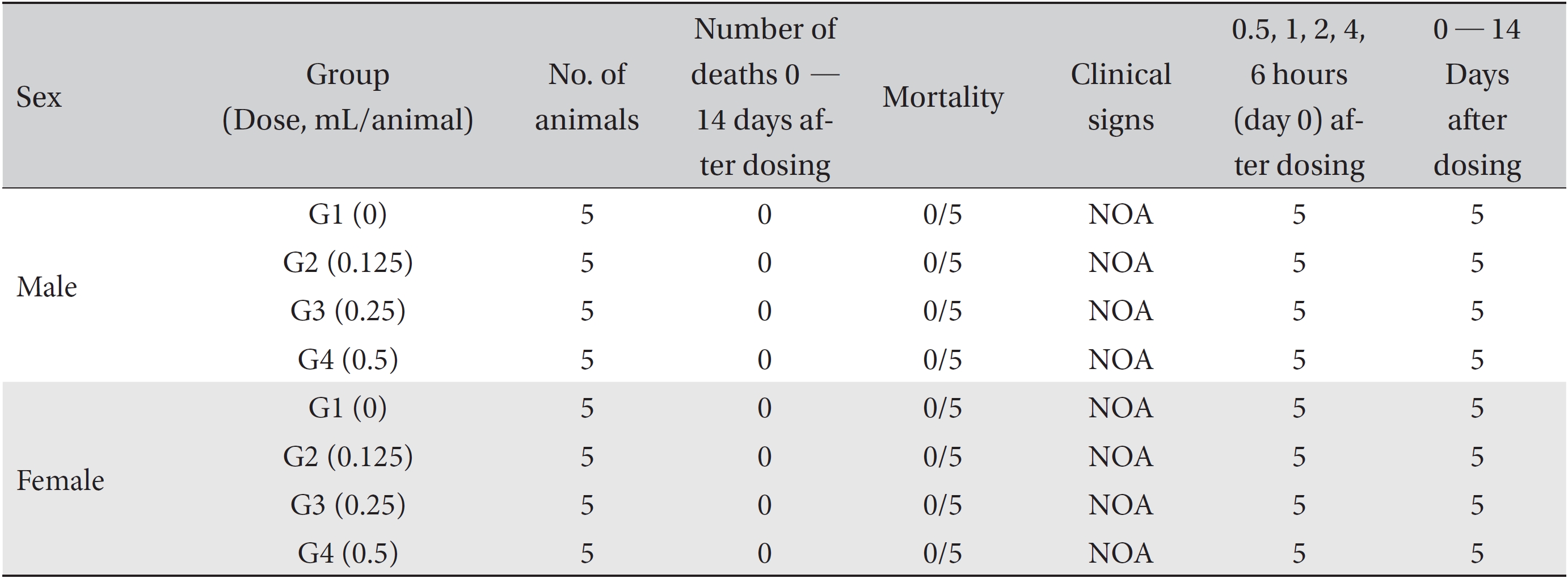
Summary of mortality and clinical signs
[Table. 3] Summary of necropsy findings
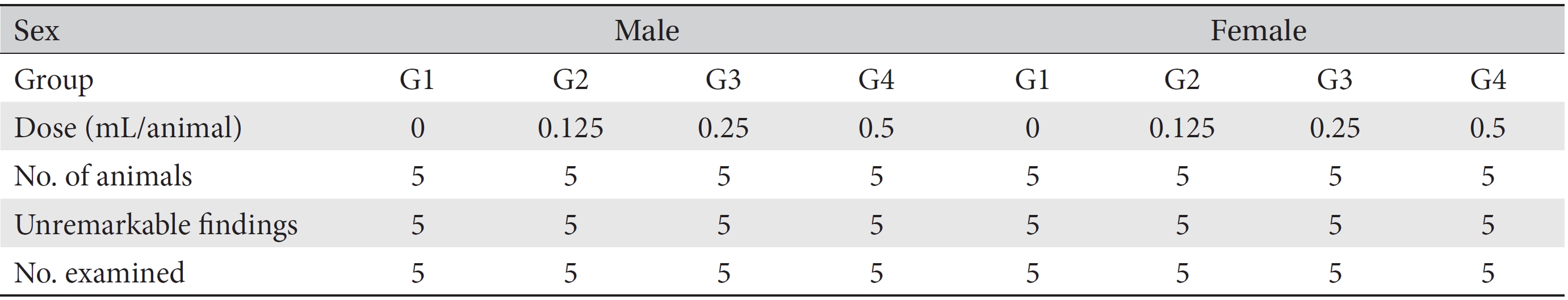
Summary of necropsy findings
[Table. 4] Summary of histopathological findings
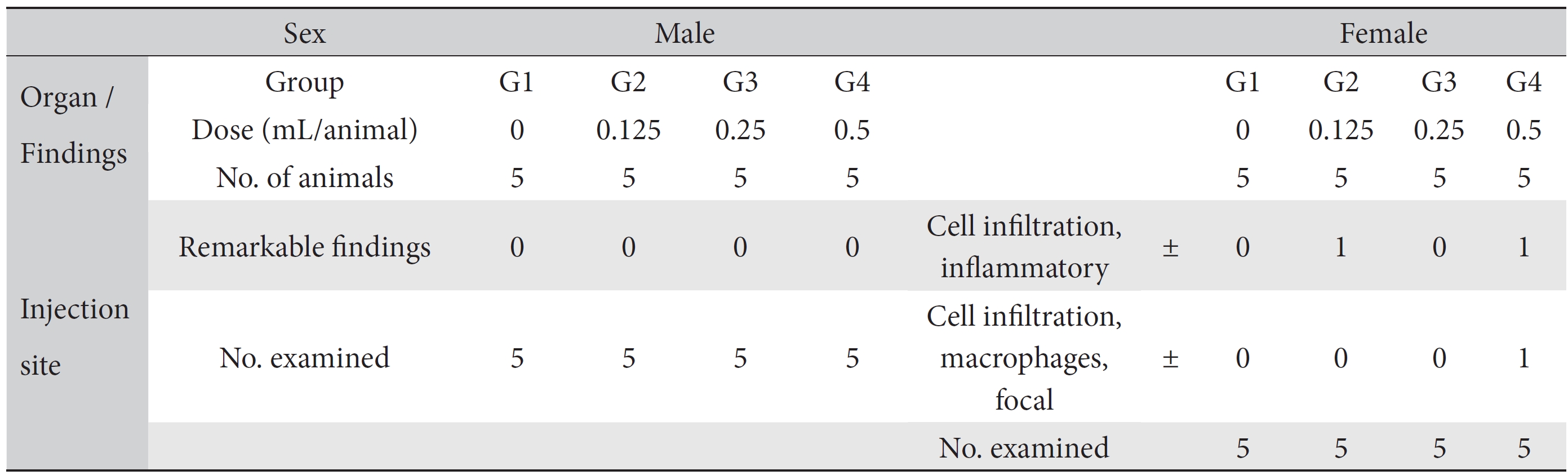
Summary of histopathological findings