During the last 13 years, efforts to find new methods for identifying the primo vascular system (PVS) have increased. Many conventional and modern methods and technologies have been utilized for the study. Confocal laser scanning microscopy [1], various types of electron microscopy, such as scanning electron microscopy (SEM), cryo-SEM, focused ion beam SEM, and high voltage transmission electron microscopy (TEM) [2-5], X-ray microtomography [6], atomic force microscopy [7], fluorescent nanoparticles [8-11], immunohistochemistry [12, 13], proteomic analyses [14], the enzyme-linked immunosorbent assay (ELISA) technique for hormone analysis [15, 16], and electrophysiological methods [17], have been employed [18]; magnetic resonance imaging (MRI) and computed tomography (CT) have also been tried [19]. The PVS is also known to be a basis for possible drug delivery pathways [18], which is of practical importance to mechanism studies for pharmacopuncture.
As the PVS is a circulatory system built by vessels and nodes [20], the utilization of Mercox is logical. Until now, the polymer Mercox has been used to fill vessels and different cavities [21-26]. As the primo vessels (PVs) are very thin and are not fixed, the direct injection of Mercox into the PVs based on the conventional procedure of corrosion casting is very difficult. One possibility for filling the PVS is to inject the Mercox through the primo nodes (PNs), but they also are not fixed, which also makes this approach difficult to apply.
In this paper we offer a new approach for using the corrosion casting polymer Mercox. We injected the polymer directly into the skin and investigated the possibility of it entering into the PVS and its distribution. This approach is completely new, can be easily applied, and can be used to fill the PVs and the PNs.
Six female Institute of Cancer Research (ICR) mice aged 8 ─ 10 weeks old were purchased from Dooyeol-Biotech, Inc (Seoul, Korea). Procedures involving the animals and their care conformed to the institutional guidelines and were followed in full compliance with current policies. The experiments completed within one or two days after the purchase of the mice. For sacrificing the mice, we injected an overdose of urethane intraperitoneally.
Red and blue Mercox (acrylic polymer in primary form, Ladd Research Industries, U.S.A.) was obtained with the catalyst for polymerization. Mercox of each color (0.5 mL of each) and 0.02 mg of the catalyst were mixed just before the injections into the skin. Disposable syringes with a 1 mL volume and with disposable needles (30 G × 1/2, Sungshin Medical Co., Korea) were used for the injections into the mice. The mixed solution, 0.1 mL, was injected subcutaneously at several acupoints on the back and the abdomen, such as CV-12, CV-16, ST-21, ST-25, BL-19, BL-25, GV-3, and GV-7. All the injections were completed within two minutes. The Mercox injected mice were kept for 30 minutes before putting them in a solution for maceration. Three percent (3%) potassium hydroperoxide (KOH, Duksan Pure Chemicals, Korea) dissolved in distilled water was prepared for maceration of the entire body of each mouse or each organ sample. In the case of the whole mouse, a partial maceration was done by putting the mouse into the potassium hydroperoxide solution for 36 hours. For the observations under the skin and for tracing the Mercox, we used a razor blade and small scissors to cut layer by layer into the deep part of the body. The instruments used for the microscopic observations were a stereomicroscope (SZX12, Olympus, Japan), an optical microscope (BX51, Olympus, Japan), and a polarizing microscope (KSM-BA3, Samwon, Korea). After the 36 hours partial maceration had been finished, the skin of the dorsal part in the mouse was cut and peeled off by using a razor and scissors from the tail to the head. This new approach of the corrosion casting can be combined with the procedure of full maceration and SEM investigation.
The advantages of the new approach are as follows: First, it uses dead animals. Second, Mercox is injected directly into the skin but not into the PVS structures. Third, for the first time, only the peripheral part of the PVS in the skin layers can be visualized. Fourth, only a partial maceration of the animal’s entire body is used in the experiment. The method makes observations possible while keeping the positions of polymerized Mercox inside the skin during the partial maceration. Otherwise, it would melt and diffuse away, thus losing the Mercox position distribution.
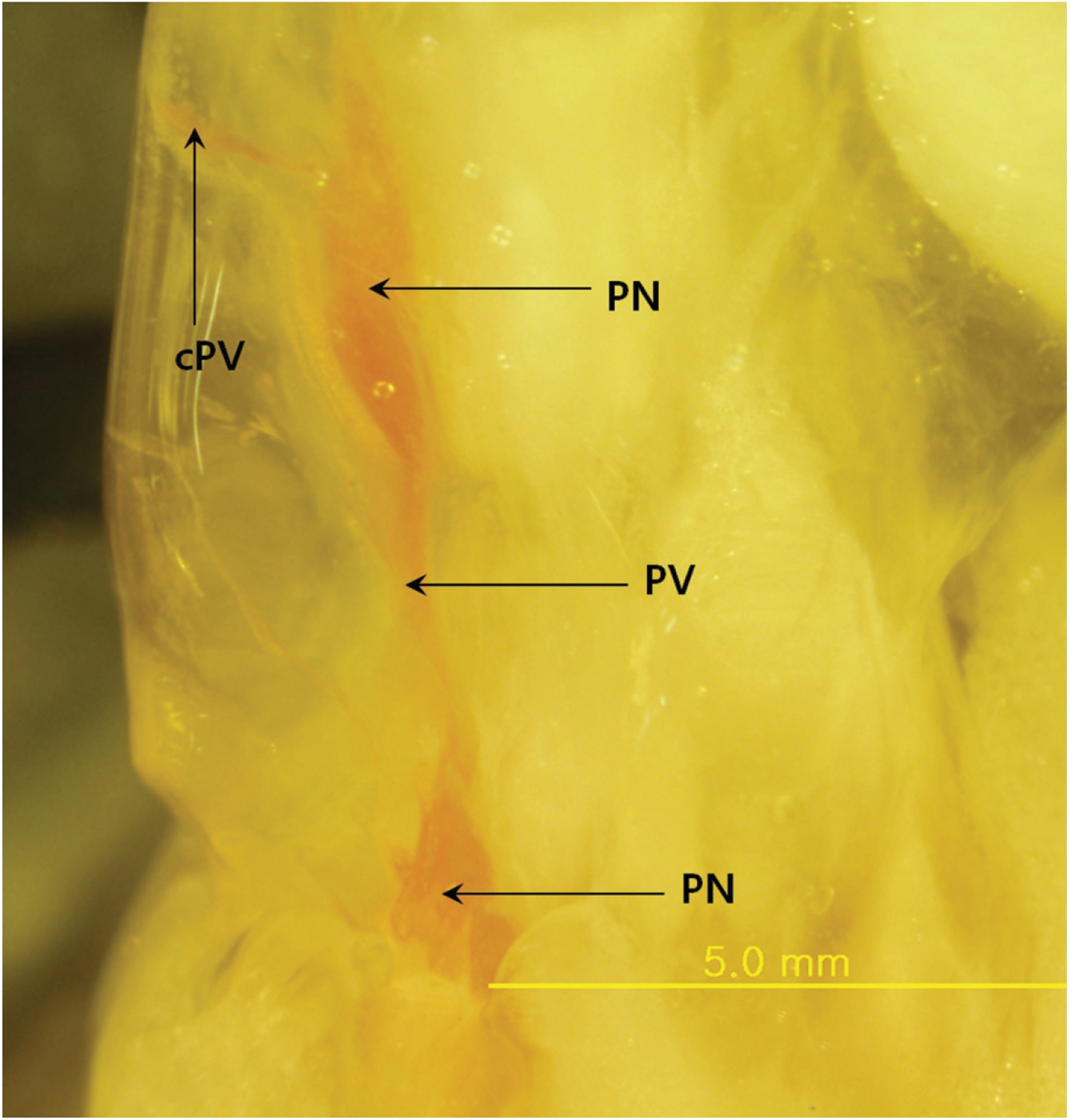
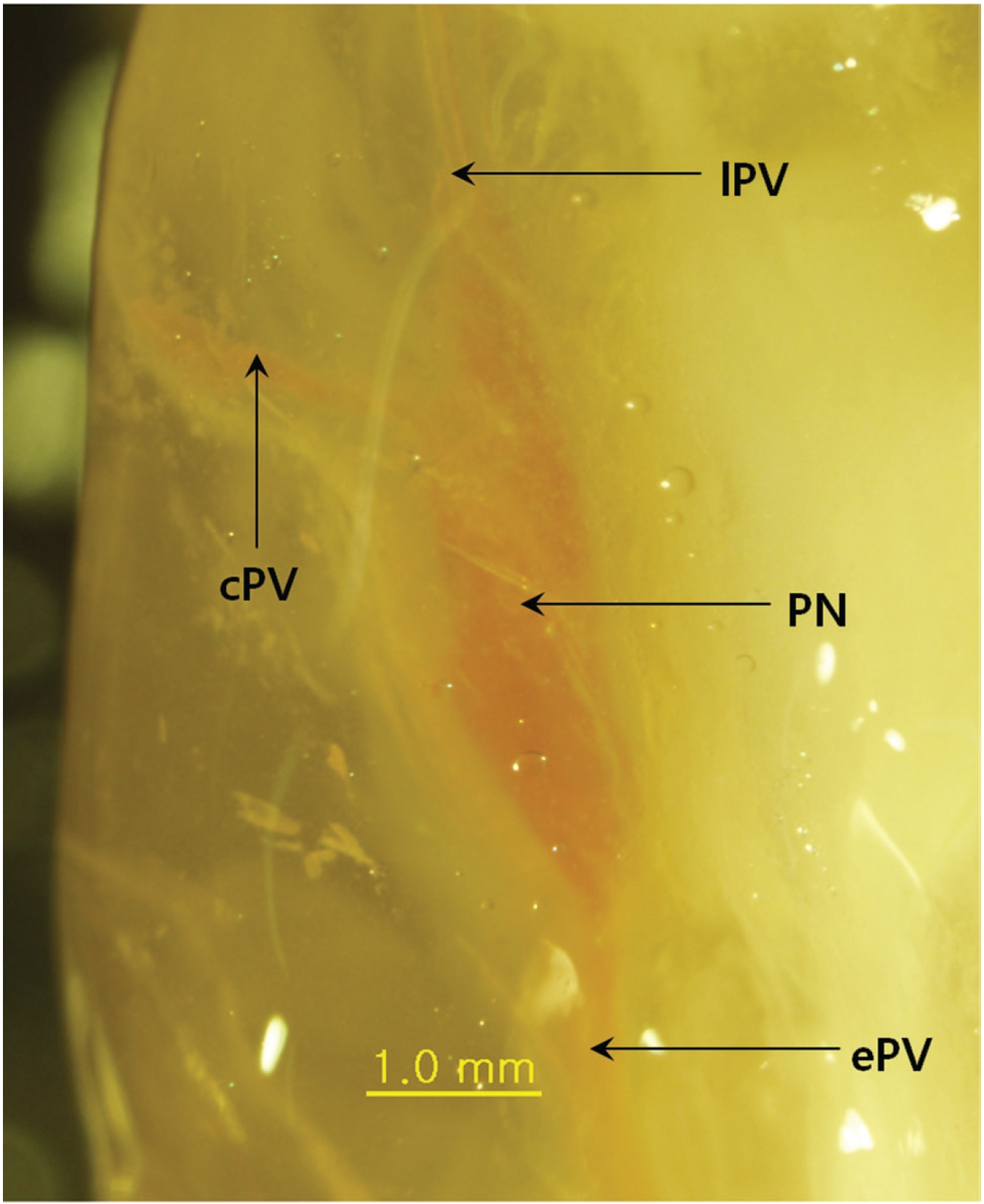
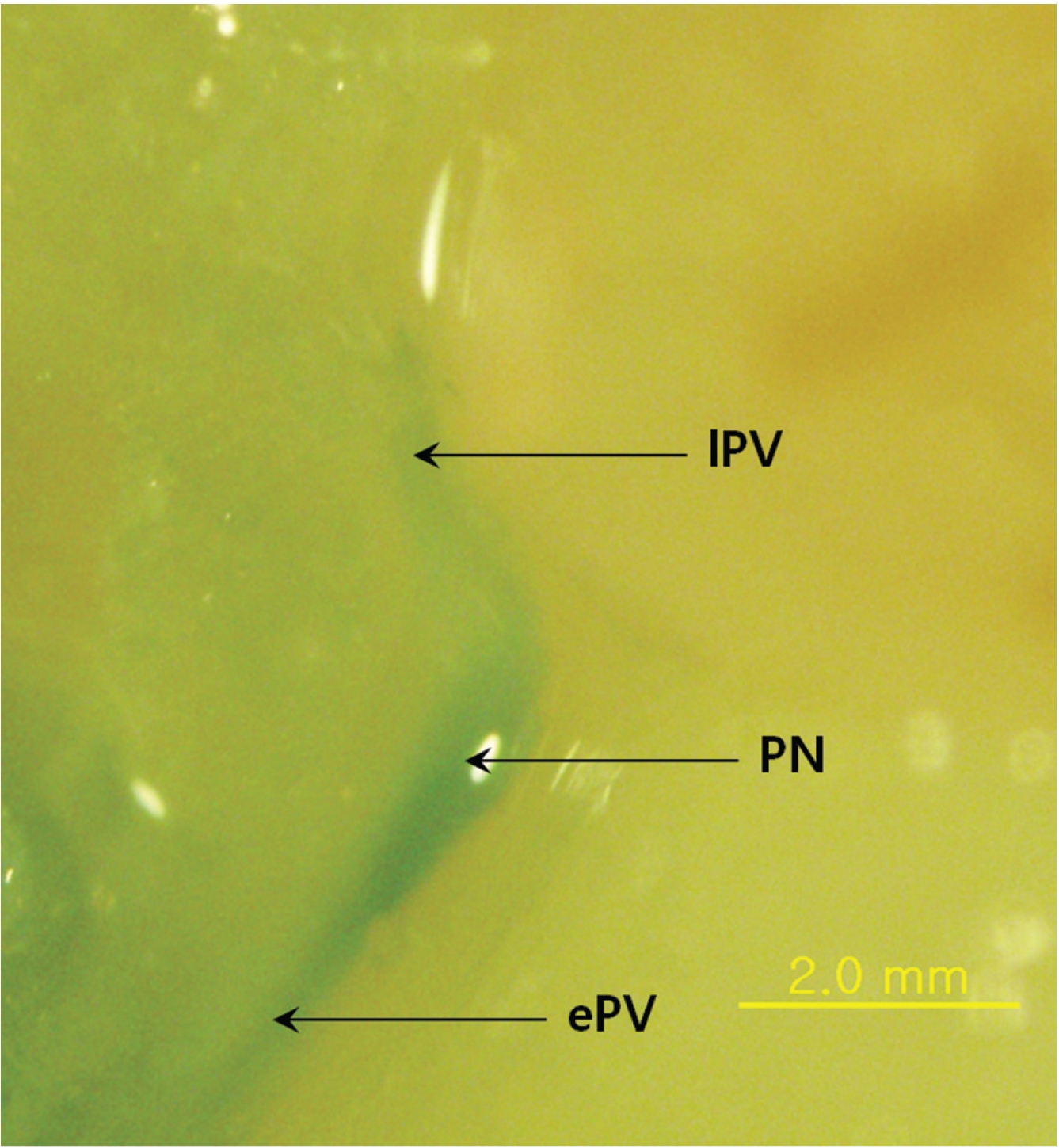
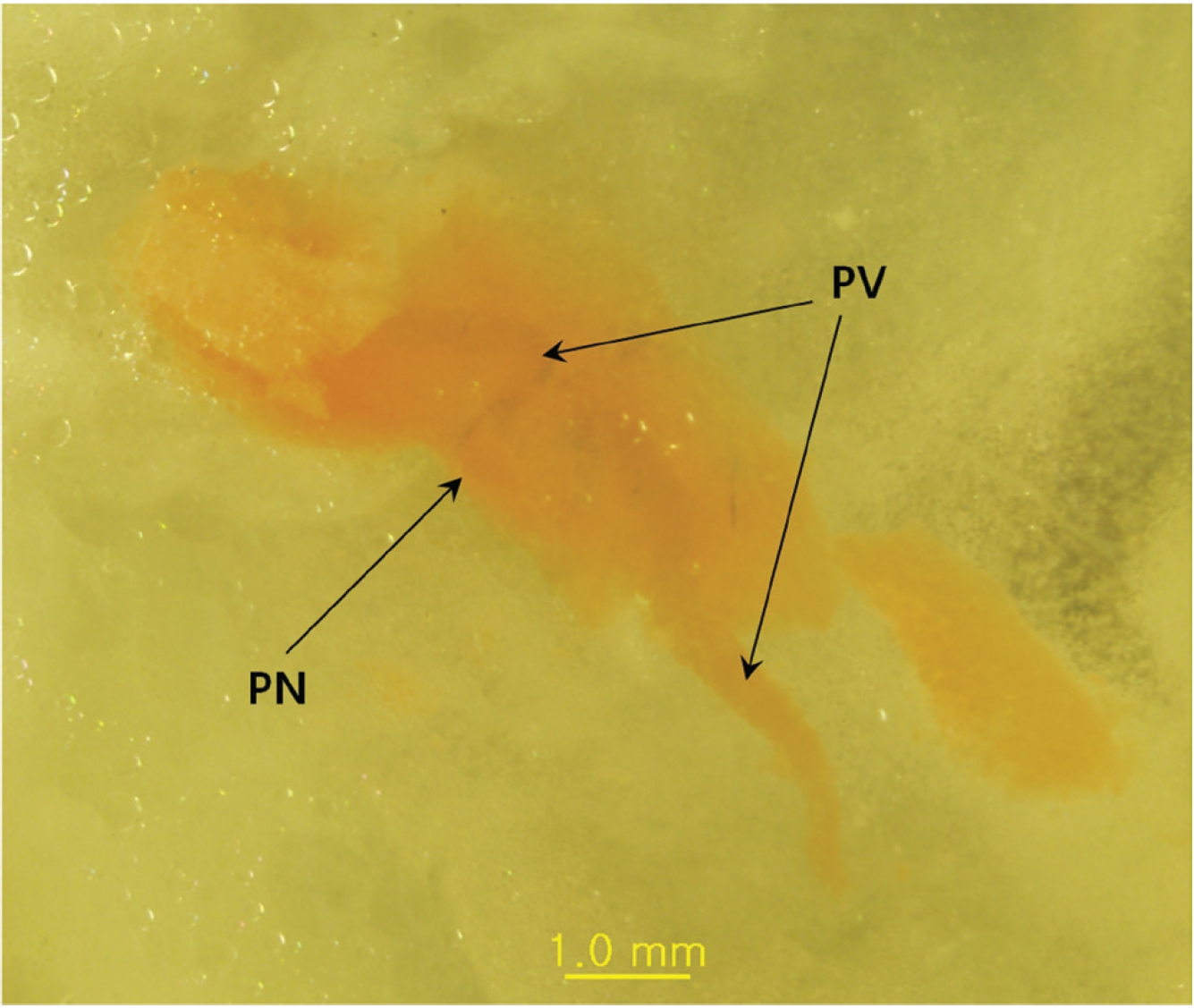
The main result of this approach is that Mercox can penetrate the primo vascular wall and fill the PVs and the PNs as a consequence, even though the material was injected outside the PVS structures and the animals were already dead. The PVs are between two PNs (Fig. 1), and the distance between the nodes is about 3 mm. In some cases, the PVs are not only entering and leaving the primo node (PN) but also a communicating primo vessel (PV) is present (Fig. 2) Communicating PVs distribute the primo liquid not only on a superficial level in the skin but go into deeper parts of the body as a communicating PV. The PNs have an elongated shape (Fig. 1) with two PVs, one on each narrow end, in most cases. The PV can also be visualized inside the PN (Fig. 3) for blue Mercox and (Fig. 4) for red Mercox), where the PV passes into the center of the PN. The shapes, sizes and locations of the PVs and the PNs are the hallmarks used to identify the PVS. Another reason to present the structures filled with polymer as the PVS is that no other structures in the skin have equal, or even similar, morphological characteristics. The PNs cannot possibly be lymph nodes because of their elongate shape, size and location, nor are they vascular or lymphatic vessels.
The PNs have different sizes depending of their locations and the animal species. The conception vessel PN of a rat is 1 ─ 2 mm in diameter [27]. Nodes in the fascia of dogs are about 0.3 ─ 1 mm [28]. On the surfaces of organs, PNs have an oval shape with a minor axis of 0.1 ─ 0.5 mm and a major axis of 0.5 ─ 1 mm, with both ends being connected to PVs of 3 ─ 6 cm in length [29]. On the surface of the endocardia in the atria and the ventricles, PNs are 100 ─ 150 μm in diameter [30]. In his review, Soh [19] concluded that the current results only suggest that an extensive fine network of the PVS exists in the hypodermis and the superficial fascia. Our results show, for the first time, that the PVS is present in the dorsal skin layers and prove that PNs and PVs exist in the hypodermal layer of mouse skin. The sizes of the PNs in mice skin are 3 ─ 5 mm along the long axis and 0.5 ─ 1.5 mm along the short axis.
The PVs found with this approach are typical for the PVS, but are not typical vascular and lymph vessels. The PVs are distributed in the skin in two different ways. When the PVs are between the PNs in the hypodermal layer of the skin, they are distributed continuously and distribute the primo liquid on the superficial layer via circulation. On the other hand, when the PNs have an additional communicating PV, that communicating PV in the PVS involves communicating with PNs deeper in the body and forms a large circular system. The latter means of communication, distributed communication, explains how the liquid and the signals are distributed in the different subsystems of the PVS. A recent study showed hemacolor staining of threadlike structures isolated from the abdominal subcutaneous tissue of rats with sizes up to 2 ─ 3 mm [31]. The larger size of the skin’s PNs may be due to the receiving of external signals.
The possibility of the polymer entering into the PVS depends on exact acupuncture points on the skin used for injection, as mentioned in “Materials and Methods”. The PVs are fenestrated [32], which allows the Mercox to penetrate into the PVS. In this case, the polymer enters into the PVS, and PVs and PNs can be visualized. The direct injection of Mercox into the skin was so successful that the method can also be applied to different internal organs [32].
The direct injection of the casting material Mercox, with modified partial maceration procedures, is a promising method for visualizing the peripheral PVS structures as PVs and PNs in the skin. The polymer Mercox, which is quite different from all other tracers used until now for studying the PVS, can penetrate through the primo vascular wall and fill PVs and PNs. The data confirm the suggestion, which was made by us in our theoretical paper [33] where we determined the peripheral part of the PVS situated in the hypodermal layer of the skin and in the superficial fascia as an external or receiving PVS, that PVs and PNs exist in the hypodermal layer of the skin. The present data suggest that the injection of pharmacological substances into the acupuncture points is the short way the activate PVS, too. The data also confirm the findings of Lim