The electric double-layer capacitor (EDLC) is an energy storage device that is superior to batteries in power density and life cycle. An EDLC stores electric energy by the adsorption-desorption of ions formed at an electric double layer between an electrode and electrolyte. Since the electrochemical performance of the EDLC changes dramatically depending on the electrode material and electrolyte, the choice of electrode material is very important [1-3].
For best performance, EDLC electrode materials should have a large specific surface area (SSA) and electrochemical stability to store lots of ions. For these reasons, porous carbon materials such as activated carbon (AC) have been widely used as the active material for EDLC electrodes. Although various ACs made of coal, coconut, timber, plant seed, etc. have been used as raw electrode materials [4-8], coal tar pitch (CTP) has been of interest as a precursor for EDLC electrode material because the AC produced from it has high a SSA and high activation yield. There have been many studies about the activation of CTP, CTP derived meso-carbon microbeads, and needle cokes [9-16].
Pore size distribution (PSD) can be changed by the activation conditions and has a huge effect on the capacity of an EDLC. Although it is conventionally known that the optimum pore sizes of an AC should be in the range of 3-5 nm (that is, meso-pores) to have a high capacitance EDLC [17,18], recent studies have proposed that micro-pores are more important than meso-pores for the capacity of an EDLC [19,20]. It has been suggested that the development of pore volumes in the range of 1-2 nm is important and governs the capacitance of EDLC with an organic electrolyte [19]. Chomila and coworkers have also reported that specific capacitance was heavily dependent on the micro-PSD and demonstrated that an anomalous increase in specific capacitance at pore sizes less than 1 nm was due to the decrease of distance between electrolyte ions and electrode materials [20].
Although previous works have mostly focused on the effect of activation conditions on the surface area and PSD, there are only a few reports about adjusting pore size based on the structure of pitch precursor [21]. Petroleum pitch (PP) can also be used as a precursor for AC. Its molecular weight distribution is normally broader and its aromaticity is lower than CTP, and there is a difference in pore formation between the two types of ACs made from CTP and PP. It was suggested that this difference was due to the large amount of compounds with low molecular weight left in PP [22]. Petrova et al. [16] found that the chemical composition of CTP precursor by means of furfural addition significantly affected the physicochemical properties of the obtained AC, such as surface area, pore structure, electrical resistance, and oxygen-containing groups on the surface. Increased furfural content facilitated the formation of a solid product characterized by high oxygen content, and the solid product was more reactive towards activation, resulting in micro-porous carbons with large surface areas.
In this study, CTP was chemically activated with KOH at different activation temperatures and times to prepare various ACs with proper PSDs. The obtained AC materials were applied as EDLC electrodes with an organic electrolyte, and their electrochemical performance was investigated. The relationship between PSD and specific capacitance was demonstrated, to provide better information on the proper pore size for high capacitance EDLC. Mixtures of CTP and PP with different compositions were also activated to prepare ACs with improved EDLC performance. Since the CTP and PP had different chemical compositions, we tried to investigate the effect of CTP/PP ratio on the pore structure and properties of the obtained AC. The obtained ACs were then fully characterized and investigated as an EDLC electrode.
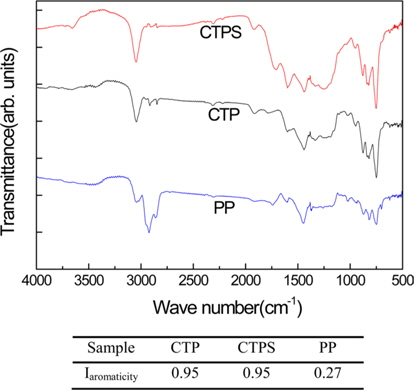
A commercial CTP provided by OCI Co., Ltd. was used as the raw material for the AC. The CTP, having a softening point of 292℃, was stabilized at 300℃ for 2 h under air in a tube furnace. A PP having a softening point of 205℃ was provided by GS Caltex Co. The stabilized CTP, and mixtures of stabilized CTP and as-received PP were activated to prepare various ACs. Fourier-transform infrared (FT-IR) spectra of the CTP and PP were obtained using a Fourier transform infrared spectrophotometer (DRS-800, Shimazu Co.). The detailed information for the CTP and PP is shown in Table 1 and Fig. 1. Potassium hydroxide (KOH, Daejung Chem. & Metals Co., Ltd., 85%) was used as the activation agent and N2 gas (JC gas, 99.99%) was flowed during KOH activation of the CTP and PP. The electrodes were prepared by mixing AC, Super-P, and poly vinylidene fluoride (PVDF, power, Aldrich) dissolved in 1-methyl-2-pyrrolidone (NMP, 99.5%, Sam Chun Pure Chem. Co., Ltd.). Coin type EDLC cells were assembled using polypropylene (PP) as a separator and 1 M tetraethylammonium tetrafluoroborate in acetonitrile (TEABF4/ACN) as an electrolyte.
[Table 1.] Properties of CTP and PP

Properties of CTP and PP
Various ACs with proper PSDs were prepared by KOH activation of the stabilized CTPs at activation temperatures in the range of 700℃-1000℃ for 1-3 h of activation time under N2 flow. The optimal mass ratio of KOH powder to CTP was selected to be 4:1. Various mixtures of CTP and PP at ratios of 10:0-1:1 were also activated to prepare ACs with different properties. The ACs obtained from the stabilized CTP and the mixtures of stabilized CTP and as-received PP were thoroughly washed with distilled water three times and dried at 110℃ in an oven for 1 day.
2.3. Characterization and electrochemical performance of AC
Pore structures of the obtained ACs were characterized by N2 adsorption/desorption measurements using an ASAP 2010 (Micromeritics) instrument. SSAs of the ACs were calculated by the Brunauer-Emmett-Teller (BET) method. The micro-pore analysis method (MP method) and the Barret-Joyner-Hallender (BJH) method were used to obtain the PSD of ACs. The crystallizability of pristine CTP, stabilized CTP, and activated CTP was determined by X-ray diffraction (XRD) measurements (Shimadzu, XRD-7000) using a Cu-Kα radiation (λ=1.54056 Å) operated at 40 kV and 30 mA.
Electrodes were prepared by mixing AC, Super-P, and PVDF at a mass ratio of 8:1:1. PVDF and Super-P were used as a binder and a conductive additive, respectively. Coin-type EDLC cells were fabricated using two symmetrical carbon electrodes. The electrodes were punched into small disks of 18 mm diameter and placed into the coin cells. A 19 mm diameter separator soaked with electrolyte solution was placed between these electrodes. The coin cells were filled with TEABF4/ACN electrolyte inside an argon filled glove box [23]. Finally, the coin cell was sealed using a coin cell crimper. The specific capacitances of the electrodes were measured by a charge-discharge tester (WBCS-3000 Battery Charge/Discharge Cycler, WonA Tech Co., Ltd.). The tests were performed in the voltage range of 0-2.7 V at a constant current of 1 A/g. Cyclic voltammetry measurements (CV, Potentiostat/Galvanostat Model 273 A, EG&G) were carried out at potential scan rates of 10 and 100 mV/s. Electrochemical impedance spectroscopy (EIS, Nova, Metrohm Autolab) measurements were performed in the frequency range of 0.01 Hz-100 kHz at open circuit potential with an AC perturbation of 5 mV [24].
3.1. Characterizations of the raw pitches
The properties of the CTP and PP are listed in Table 1. Although the softening point and C/H ratio of the CTP were higher than those of PP, the differences in their solubility in toluene and quinoline were even more obvious. The huge difference in solubility between two pitches could be explained by the higher content of aromatic compounds in CTP. To analyze their aromaticity and chemical structure, FT-IR spectra of the two types of pitches are presented in Fig. 1. Some functional groups of CTP and PP were observed to be aromatic (3050, 1161, 1505, 1052, 872, 811, 750, and 438 cm-1) and aliphatic (2955, 2920, 2855, and 1450 cm-1) groups. Specifically, we can see the existence of aromatic C-H stretch at 3050 cm-1 and the stretching of aromatic C=C at 1611-1470 cm-1 [25-28]. Compared to the PP, the CTP and stabilized CTPS had noticeable increases of absorption at 3050 cm-1 and decreases of absorption at 2920 cm-1 but there were no significant differences between CTP and CTPS. The aromaticity index of various pitches was calculated by Eq. (1) made by Guillén et al. [25].
The aromaticities of CTP, CTPS and PP were evaluated to be 0.95, 0.95, and 0.27 respectively.
3.2. Optimization of activation conditions for CTP
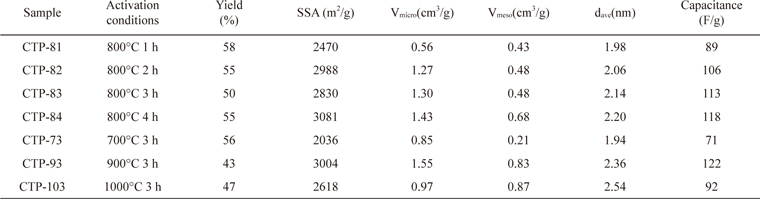
The properties of the ACs prepared from CTP by KOH activation at 700℃-1000℃ for 1-4 h are summarized in Table 2. With increasing activation time at 800℃, the specific capacitance of the AC increased, up to 4 h. As the activation temperature was varied from 700℃ to 1000℃ for 3 h of activation time, the specific capacitance of the AC increased up to 900℃ but it reduced with further increase of activation temperature. Thus, the activation treatment of 900℃ for 3 h was selected as having the optimal activation conditions, and provided the largest specific capacitance of 122 F/g. The specific capacitance was calculated by a charge-discharge test in a two-electrode system using Eq. (2).
[Table 2.] Properties of activated carbons prepared from CTP at different activation conditions
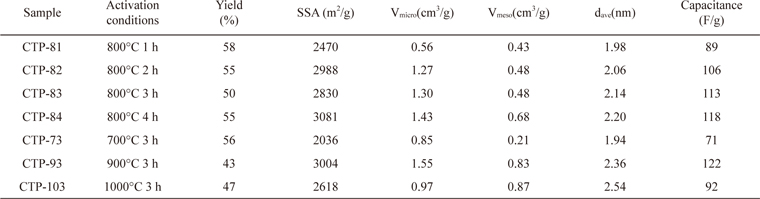
Properties of activated carbons prepared from CTP at different activation conditions
Here C is the specific capacitance, I the discharge current, Δ
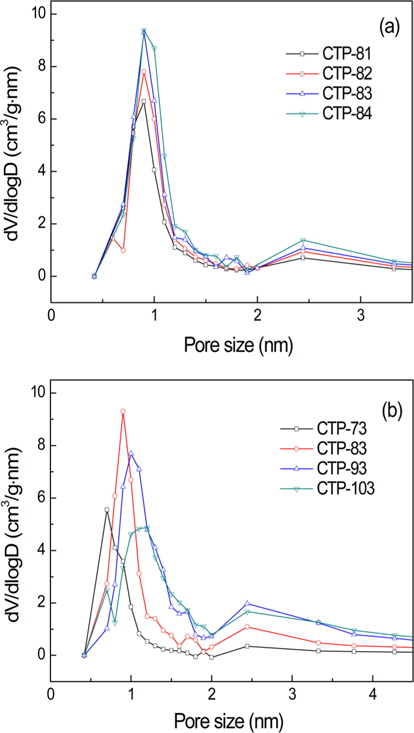
Fig. 2 shows the PSD of ACs fabricated from CTPs activated at 800℃ for 1-4 h (a) and at 700℃-1000℃ for 3 h (b). It can be seen that with increasing activation time at 800℃, the pore volumes of ACs with pore sizes from 1 to 2 nm tend to increase. The specific capacitance of the ACs also increased with increasing activation time as shown in Table 2. As the activation temperature increased from 700℃ to 1000℃ with 3 h of activation time, the pore volumes of the AC with pore sizes from 1 to 2 nm increased up to 900℃ but it reduced at 1000℃ activation. Since the specific capacitance of the electrodes and the PSD of the ACs exhibited a similar trend according to the activation conditions, it was demonstrated that the pore volume of ACs with pore sizes from 1 to 2 nm was suitable for high electrode performance in the organic electrolyte EDLC system.
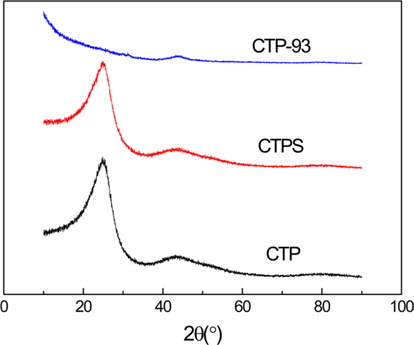
To confirm the change of crystal structure based on the AC preparation process, XRD profiles of as-received CTP, stabilized CTPS, and CTP activated at 900℃ for 3 h (CTP-93) are presented in Fig. 3. Both the CTP and CTPS had a diffraction peak (002) of graphite at 2θ = 25°, but it disappeared for CTP- 93, indicating the disintegration of the graphitic crystal structure and the formation of a well-developed pore-structure through the KOH activation of CTP [9].
3.3. Properties of a mixture of CTP and PP
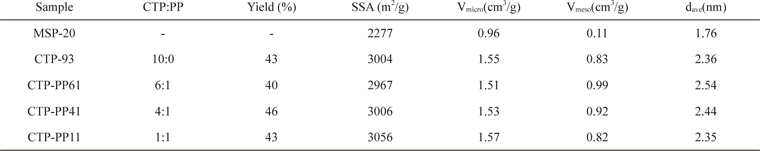
In order to examine the differences in physical and electrochemical properties of ACs according to the chemical composition of their precursor pitches, various mixtures of CTP and PP were activated. Table 3 presents the properties of ACs prepared from different mixtures of CTP and PP with activation conditions of 900℃ and 3 h. For comparison, the property of a well- known commercial activated carbon, MSP-20 (Kansai Coke & Chemicals Co., Japan), is also shown. In general, the pitch-based ACs had higher SSAs and pore volumes than those of MSP-20.
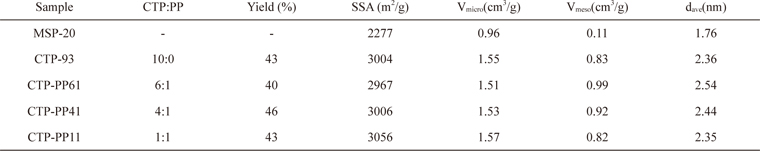
Properties of commercial AC (MSP-20), and ACs prepared from different mixtures CTP and PP at activation conditions of 900℃ and 3 h
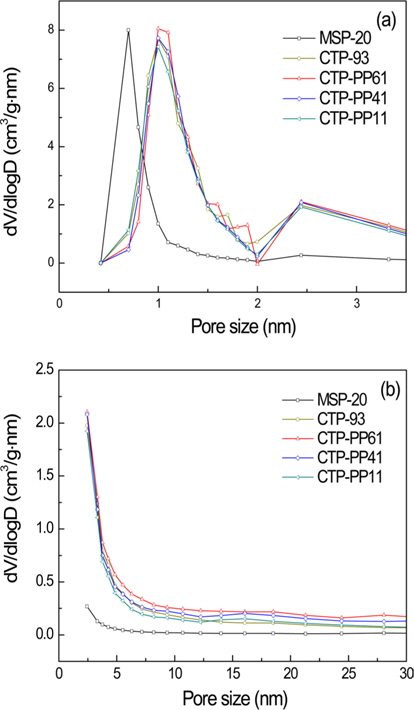
Compared to the CTP-93, the AC from CTP-PP11 had a little bit increased SSA and similar micro- and meso-pore volumes. For the ACs with increased CTP contents such as CTP-PP41 and CTP-PP61, the SSA and micro-pore volume decreased a little bit, but meso-pore volumes and average pore diameter increased. The AC prepared from CTP-PP61 had the highest pore volume and average pore diameter. Fig. 4 shows the PSDs of MSP-20, and the ACs from various mixtures of CTP and PP. In the micro-pore analysis of Fig. 4a, MSP-20 had large PSD in the range of 0.5-1 nm, while the pitch-based ACs had large PSD in the range of 1-2 nm. For the meso-pore analysis of Fig. 4b, the difference of PSD between MSP-20 and the pitch-based ACs is more obvious. This large PSD could help to achieve outstanding electrochemical performance at high current rates.
Although the PSDs of ACs prepared from various mixtures of CTP and PP were very much similar to each other, the pore volume less than 1 nm declined but the pore volume sizing from 1 to 2 nm as well as the meso-pore volume increased with the increased CTP contents in the pitch mixtures, showing a maximum with CTP-PP61.
As shown in Table 1 and Fig. 1, the chemical composition of PP is quite different from that of CTP. Compared with CTP, the molecular weight distribution of PP is broader and the aromaticity is lower. Since PP has a larger amount of compounds with low molecular weight and a lower content of aromatic hydrocarbon, PP might have some harmful effect on the formation of graphite crystals during the heat treatment of the activation process. The less graphitic carbon prepared with a high content of PP would be more reactive towards activation by KOH, generating the more developed pore-structure.
However, the control of porosity during KOH activation was not easy with just the PP precursor. Thus, a mixture of PP and CTP was used to provide structural deviation in the precursor mixture. Activation means the removal of less graphitic carbons by selective oxidation. So, the deviation in the graphitic structure would allow easier control of porosity depending on the extent of activation. A more favorable structural condition for the proper pore-size distribution could be obtained with a mixture of CTP and PP. Since the pore volume of ACs with pore sizes from 1 to 2 nm was suitable for high electrode performance in our EDLC system, the AC prepared from CTP-PP61 could be expected to exhibit the highest electrochemical performance.
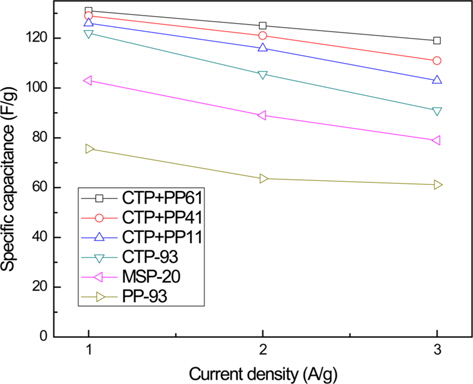
Fig. 5 shows the specific capacitance of MSP-20, and the ACs prepared from various mixtures of CTP and PP, measured by a charge-discharge test in the voltage range of 0-2.7 V and plotted as a function of current density. The specific capacitance of ACs prepared from the mixtures of CTP and PP was better than that from CTP only, and MSP-20. Although the specific capacitance of the AC electrodes fabricated from CTP only, and from mixtures of CTP and PP, was not significantly different at a current density of 1 A/g, the AC electrodes from CTP and PP mixtures showed outstanding specific capacitance at higher current rates. In particular, CTP-PP61 had the highest specific capacitance of 132 F/g at a current density of 1 A/g, and the specific capacitance remained above 90% at a high current density of 3 A/g. It was demonstrated that the high specific capacitance could be attributed to the increased micro-pore volume of ACs with pore sizes from 1 to 2 nm, and the high power density could be attributed to the increased meso-pore volume.
EIS analysis was conducted to explore the internal resistances of EDLCs using various ACs as electrode material. In an EDLC system, the response to a sinusoidal potential E will be a sinusoidal current I at the same frequency with a shift in phase. The resulting impedance Z is the following complex number.
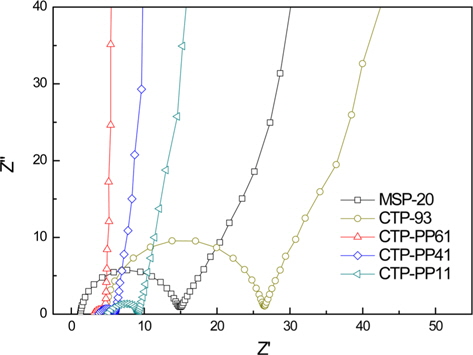
Fig. 6 shows the EIS of different AC electrodes prepared from the mixtures of CTP and PP, and CTP-93 in the frequency range of 0.01 Hz-100 kHz. The Nyquist graphs not only present the resistance value but also reflect the dynamic process needed to form electric double layers. The Nyquist plots for various samples were similar to each other. They were composed of a semicircle region at high frequencies, a sloping linear region at medium frequencies, and a nearly vertical line at low frequencies [29].
In a Nyquist plot, there are two resistances, solution resistance (Rs) and internal contact resistance of the electrode (Rc). Rs comes from the bulk solution resistance depending on the electrolyte conductivity and the separator material. Rc can be separated into two components: the electronic and the ionic resistances. The electronic resistance includes the intrinsic electronic conductivity of the carbon particles, the electronic contact between particles, and the contact between the active layer and the current collector. The ionic resistance is the electrolyte ionic resistance inside the pores of the electrode depending on the electrolyte conductivity and pore structure of the electrode materials. Rs and Rc can be determined by the beginning and terminal points of a semicircle in the Nyquist plot. The internal resistance of the cell, Rint, is estimated from the intersection between the straight section of the Nyquist plot and the X-axis [30]. The impedance data for the various samples are listed in Table 4.
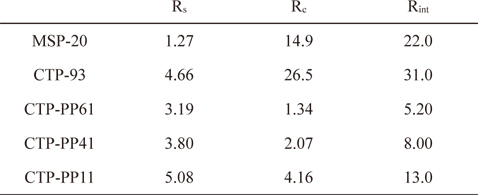
Impedance results of commercial AC (MSP-20), and ACs prepared from different mixtures of CTP and PP
The semicircle terminal points for AC electrodes prepared from the mixtures of CTP and PP were much lower than those for CTP-93 and MSP-20 due to their well-developed pore structures including the larger meso-pore volumes. With increasing CTP content, Rs decreased, showing a minimum of 1.34 Ω with CTP-PP61. The CTP-PP61 sample also had a minimum Rint of 5.20 Ω.
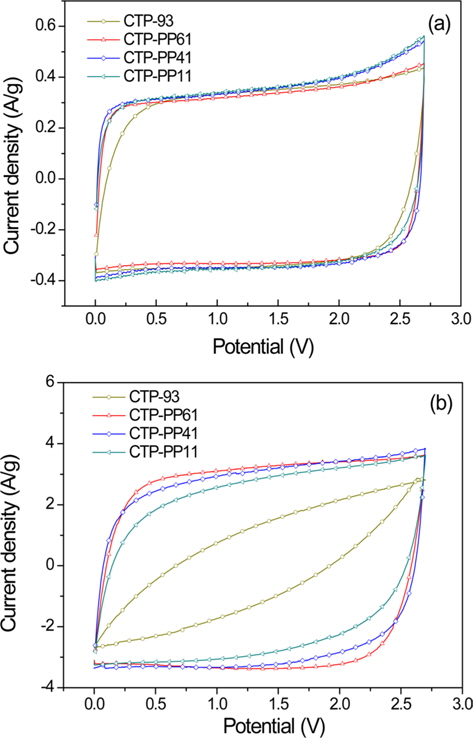
CVs of AC electrodes prepared from the various mixtures of CTP and PP are presented in Fig. 7. As shown in Fig. 7a, CV curves for all five samples showed a rectangular, symmetric, and reversible shape in the voltage range of 0-2.7 at a scan rate of 10 mV/s. As the scan rate increased to a high scan rate of 100 mV/s, the CV curve for CTP-93 changed into a rugby ball shape and the specific capacitance decreased dramatically. However, the shape of the CV curve and their specific capacitance for the mixtures of CTP and PP remained at a high scan rate of 100 mV/s. The excellent capacitance behavior of pitch-based ACs at high scan rates indicates that their equivalent series resistance was very low.
Various ACs were prepared by KOH activation of CTP and from mixtures of CTP and PP for application as the electrodes of EDLCs. For ACs prepared from CTP only, the optimal activation conditions were determined to be a CTP/KOH ratio of 1:4, activation temperature of 900℃, and activation time of 3 h. The obtained ACs showed increased PSD in the range of 1 to 2 nm and the specific capacitance of 122 F/g in a two-electrode system with an organic electrolyte.
In order to improve the performance of the EDLC electrode, mixtures of CTP and PP were activated at the optimal activation conditions previously determined for CTP. By introducing a small amount of PP into the CTP, the obtained AC electrodes showed improved specific capacitance as well as outstanding power density. It was demonstrated that the high specific capacitance can be attributed to the increased micro-pore volume of ACs with pore sizes from 1 to 2 nm, and the high power density can be attributed to the increased meso-pore volume.