Graphene has elicited great interest since it was discovered by Andre Geim and Konstantin Novoselov in 2004 [1,2]. On the basis of its unique electrical, optical, thermal, and mechanical properties it is desirable for a broad range of high-tech applications in flexible transparent electronic devices, supercapacitors, batteries, composites, and flexible transparent displays and sensors [3]. Top-down and bottom-up strategies, such as mechanical exfoliation of graphite, chemical reduction of graphene oxide (GO), unzipping of carbon nanotubes, chemical vapor deposition, and epitaxial growth, have been developed to produce graphene [4,5]. Among these strategies, the chemical reduction of GO is the most attractive owing to its low fabrication cost and capability of mass production [6]. In terms of the feasibility of this method, however, chemical reduction has been a critical issue, and it has been accomplished by various methods, including the use of chemical reducing agents, thermal and solvothermal treatment, electrochemical and photocatalytic reduction, and radiationinduced chemical reduction [7-9].
Radiation-induced chemical reduction is considered a desirable route for reducing GO, in terms of environmental friendliness, efficiency, cost, and scalability [10]. In this light, radiation-induced chemical reduction of GO suspensions in aqueous and non-aqueous solvents recently has been studied [11-13]. However, radiation-induced chemical reduction of GO with various functional groups has not yet been explored.
In this study, the preparation of sulfonated reduced graphene oxide (SRGO) by the sulfonation of GO followed by the irradiation of sulfonated graphene oxide (SGO) with γ-rays is described. The prepared SRGO has been characterized in terms of its optical, chemical, thermal, and electrical properties.
Graphite powder (SP-1) was supplied from Bay Carbon (Bay City, MI, USA). Concentrated sulfuric acid (H2SO4), potassium peroxodisulfate (K2S2O8), phosphorus pentoxide (P2O5), potassium permanganate (KMnO4), hydrogen peroxide (H2O2), hydrochloric acid (HCl, 0.1 N), ethanol (EtOH), sulfanilic acid (C6H7NO3S), sodium hydroxide (NaOH), and sodium nitrite (NaNO2) were purchased from Aldrich Company. All chemicals in this study were used as received.
2.2. Preparation of SGO by aryl diazonium reaction
A 20 mL GO suspension (10 mg/mL) in deionized water prepared from graphite powder by the well-known modified Hummer’s method was added to a three-necked round flask [11,14]. To prepare an aryl diazonium salt solution, sulfanilic acid (500 mg) and sodium nitrate (200 mg) were dissolved in 10 mL of 2 wt% NaOH solution at 50℃. The resulting homogenous solution was added into 20 mL of 0.1 N HCl solution in an ice bath with stirring [15]. After reaction for 15 min, the resulting aryl diazonium salt solution was added dropwise into a three-necked round flask containing a 20 mL GO suspension and was vigorously stirred for 4 h in an ice bath. To obtain SGO, the resulting solution was centrifuged and rinsed with deionized water more than five times. The obtained SGO was readily dispersed in EtOH at a cogeneration of 1 mg/mL.
2.3. Preparation of SRGO by γ-ray irradiation-induced chemical reduction
To prepare SRGO, 80 mL aliquots of as-prepared SGO (1 mg/ mL) solution in EtOH were poured into 100 mL glass bottles, which were then sealed with rubber sept, and finally purged with nitrogen gas to eliminate the existing oxygen. The bottles containing the SGO solution were irradiated with γ-rays at room temperature from a 60Co source at the Advanced Radiation Technology Institute (ARTI) of the Korea Atomic Energy Research Institute (KAERI). The total absorbed doses ranged from 10 to 50 kGy, and the dose rate was 5 kGy/h. The resulting solution was then isolated by filtration through a 0.45 μm polytetrafluoroethylene membrane filter and dried in a vacuum oven at 60℃ for 24 h.
Ultraviolet-visible (UV-vis) spectra were recorded on a UV-Vis spectrophotometer (Scinco S-3100, Korea). A Fourier transform infrared (FT-IR) spectroscopic analysis was performed using an FT-IR spectrometer (Varian 640, Australia). An X-ray photoelectron spectroscopic analysis (XPS) was carried out using a MultiLab 2000 X-ray photoelectron spectroscope (Thermo Electron Corporation, England). A thermogravimetric analysis (TGA) was conducted on a Q600 analyzer (TA Instruments, USA) in a temperature range of 50℃ to 600℃ at a heating rate of 5℃/min under a nitrogen atmosphere. The electrical conductivities of 100 nm-thick SRGO films measured with an AlphaStep IQ surface profiler (KLA Tencor, USA) were measured through a MCP-T610 four-point probe conductivity measurement system (Misubishi LORESTA-GP).
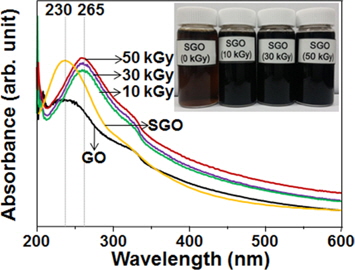
The optical properties of the GO, SGO, and SRGO samples prepared at various absorbed doses were investigated through a UV-vis analysis, and the results are shown in Fig. 1. The GO and SGO specimens exhibited an identical absorption peak at 230 nm, indicating that the optical properties of the GO were not significantly affected by the aryl diazonium reaction-based sulfonation. On the other hand, the absorption peak of all SRGO samples prepared at the given absorbed doses was present at a longer wavelength of 265 nm in comparison to that of the SGO [16]. In addition, as shown in the inset (Fig. 1), the brown solution of the SGO in EtOH was transformed to a dark color after γ-ray irradiation. This indicates that SRGO was successfully prepared by γ-ray irradiation-induced restoration of the π-conjugation system in the SGO.
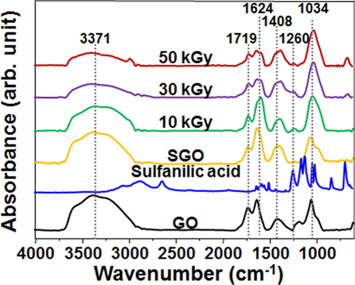
The chemical structures of the GO, SGO, and SRGO samples prepared at various absorbed doses were investigated by a FT-IR analysis, and the results are shown in Fig. 2. The characteristic peaks of the GO were observed at 3371 (-OH), 1719 (C=O in carboxylic acid), 1624 (absorbed H2O or aromatic C=C), 1408 (C-OH), and 1034 (C-O) cm-1, respectively. In the SGO spectrum, the characteristic peak corresponding to the chemical structure of sulfanilic acid was clearly observed at 1260 cm-1 (O=S=O), although the peaks for the amide and phenyl groups hidden by the GO could not be observed [17]. On the other hand, the oxygen-containing functionalities such as -OH, C=O, and C-OH were reduced with an increase in the absorbed dose [18]. Furthermore, the [O]/[C] and [S]/[C] atomic ratios of the GO, SGO, and SRGO samples obtained through an XPS analysis revealed that the [O]/[C] and [S]/[C] ratios of the SGO were higher than those of the GO, confirming successful sulfonation, and those of the SRGO decreased with an increase in the absorbed dose in comparison to those of the SGO [19]. This implies that SGO was successfully prepared by the sulfonation of GO, and the resulting SGO suspensions in EtOH were effectively reduced by γ-ray irradiation-induced deoxygenation, resulting in the formation of SRGO.
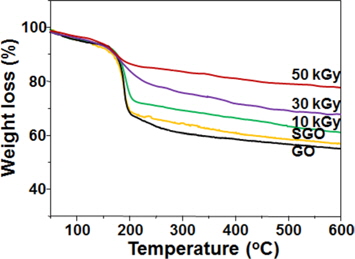
The thermal decomposition curves of the GO, SGO, and SRGO samples prepared at various absorbed doses are shown in Fig. 4. As shown in the GO and SGO curves, the SGO showed a similar tendency to the characteristic decomposition curves of the GO owing to the small extent of sulfonation. On the other hand, the SRGO exhibited a dose-dependent decrease of its weight in a temperature range of 150℃ to 250℃ in comparison to that of the SGO, and this is ascribed to the elimination of thermally-liable oxygen containing functionalities during γ-ray irradiation [20].
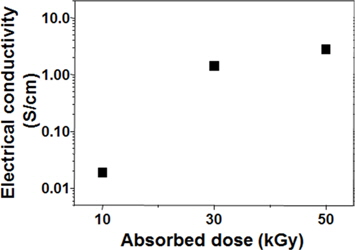
The electrical conductivities of the SRGO samples prepared at various absorbed doses were measured using a four-point probe, and the results are shown in Fig. 5. The electrical conductivities of GO and SGO could not be obtained in this measurement system, the lowest limit of which is 10-7 S/cm. On the other hand, the electrical conductivity of the SRGO was increased up to 2.94 S/cm as the absorbed dose was increased. It was thus confirmed that SRGO was successfully produced by γ-ray irradiation- induced reduction of SGO.
The preparation of functionalized graphene (SRGO) by the sulfonation of GO followed by γ-ray irradiation-induced chemical reduction was successfully demonstrated in this work. The results of UV, FT-IR, XPS, and TGA analyses revealed that SGO suspensions in EtOH prepared by the sulfonation of GO with aryl diazonium salt were successfully reduced to SRGO by γ-ray irradiation-induced deoxygenation, and the optical, chemical, and thermal properties of the resulting SRGO were dependent on the absorbed doses. Furthermore, the electrical conductivity of the SRGO was increased up to 2.94 S/cm with an increase in the absorbed dose. This method can be used in the mass-production of SRGO suitable for various applications including composites, coating, paints/inks, biomaterials, and electronic materials.
![[O]/[C] (a) and [S]/[C] atomic ratios (b) of graphene oxide (GO), sulfonated GO (SGO), and sulfonated reduced GO (SRGO) prepared at absorbed doses of 10, 30, and 50 kGy, determined by an X-ray photoelectron spectroscopic analysis.](http://oak.go.kr/repository/journal/15587/HGTSB6_2015_v16n1_41_f003.jpg)