



전도성 고분자를 이용한 새로운 기능성 재료의 개발은 에너지, 환경, 나노 기술 분야 발전에 크게 기여할 것으로 기대를 모으고 있다. 최근에는 전도성 고분자에 기능성 도펀트(functional dopant)를 삽입하여 고성능화에 초점을 맞춘 연구들이 많이 수행되고 있다. 본 총설은 새로운 전도성 고분자 합성의 관점으로 쓰여진 다른 문헌들과 달리, 삽입되는 기능성 도펀트의 역할과 응용 분야를 중심으로 서술하였다. 대표적인 기능성 도펀트의 종류로는 산화환원 활성(redox-active) 분자, 카본나노물질, 바이오물질, 킬레이팅(Chelating) 분자 등이 있으며, 각각의 도펀트의 고유한 특징에 따라 베터리, 수처리용 분리막, 센서 등 다양한 분야에 활용될 수 있다. 본 총설에서는 각각의 기능성 도펀트가 첨가 되었을 때 장점과 응용 방향에 대해 살펴 보고, 전도성 고분자의 안정성 향상을 위한 방법과 고려해야 할 점들에 대하여 제안하고자 한다.
Heeger, MacDiarmid and Shirakawa were awarded the Nobel Prize in Chemistry in 2000 for their seminal discovery and following development of “plastics that conduct electricity”. The initial discovery of conducting polymers (CP) occurred in 1977, where it was shown that polyacetylene (pAcetyl) doped with iodine was an electrical conductor[1,2]. The discovery of other conductive polymers followed, including polypyrrole (pPy), polythiophene (pThio) and polyaniline (pAni)[3-5]. Several new concepts (polarons, bipolarons and solitons) were introduced to explain how ionic-charge carriers were responsible for metallic conductivity in these materials.
All conducting polymers consist of a conjugated system of single and double bonds that alternate along a planar or nearplanar sequence. If the electrons within this system were completely delocalized, all of the bonds lengths along the polymer backbone would be identical and the polymer would exhibit metallic behavior (i.e., no band gap between the valence band (VB) and conduction band (CB)). Structural distortions caused by strong coupling between the -electrons and the vibrational modes (phonon) of the polymer backbone, however, remove the singularity in bond length and consequently limit delocalization. As such, these materials behave as wide band-gap semiconductors. Doping (predominantly p-doping) of these polymers is an essential process to making them electrically conductive[5,6].
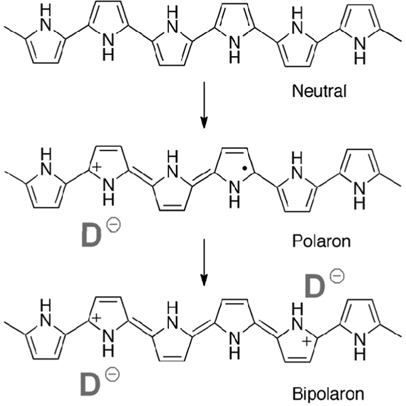
Doping of CPs has a tremendous effect on the electrical properties of these materials by modifying their band gap. Polymer backbone of CP is oxidized (p-doped) or reduced (n-doped) when dopants are introduced. Alternatively, dopants are incorporated as counter ions into CP when the CP is oxidized or reduced. The charges introduced into CPs during the doping processes combine with the distorted structure of the CP to form an ionic complex commonly referred to as a polaron or bipolaron (e.g., pPy) or solitons (e.g., pAcetyl). (Figure 1) The energy states of the ionic complexes resides in the band gap between VB and CB. Increasing the doping level intensifies the interaction between ionic complexes to form bands of electronic states. Eventually, the bands overlap the VB and CB to yield a continuous band structure or the metallic one.
Conducting polymers have been doped with a wide spectrum of compounds. For example, small anions (Cl-, ClO4-, and BF4-), medium-sized anions (p-toluenesulfonate (TS), dodecylbenzenesufonate (DBS)), and polymeric anions (polystyrenesulfonate (pSS)) have been used as p-type dopants in CPs. Anionic dopants neutralize the polycationic backbone of CPs (e.g., one positive charge for every four monomeric units in pPy). Doped CPs can be used as electrodes in electrochemical devices and as matrixes to entrap molecules, providing a conductive, porous environment. They are reduced to their insulating state at negative potentials, releasing their dopants or uptaking counterions to compensate for charge imbalance with trapped anionic dopants. A change in color (electrochromism) or volume (actuator) of the CP film accompanies the reversible redox reactions.
To add extra functions to the basic characteristics of CPs, two different strategies have been employed. The first strategy is to modify monomers by adding pendant functional moiety[7-9]. Pyrroles having electroactive species such as ferrocene on their nitrogen through covalent bonds is one example. The electroactivity of the modified monomers was transferred to their polymeric films[7]. As an alternative and simpler strategy, functional dopants can be incorporated into CPs without any modification on monomers. The characteristic properties of dopants are introduced into CPs after polymerization. Conventional monomers of CPs can be used, thereby eliminating complicated synthetic procedures of monomers. The latter strategy, “Incorporation of functional dopants into CPs”, has several merits from the point of view of dopants. First, molecules of interest (MOIs) are easily immobilized within a conductive environment. Second, MOIs are dispersed homogeneously within CPs in a molecular level at a fixed ratio. Third, MOIs can be easily patterned via electrodeposition. Fourth, MOIs doped in CPs can be investigated even in solutions in which they are insoluble.
2. Types of functional dopants and their applications
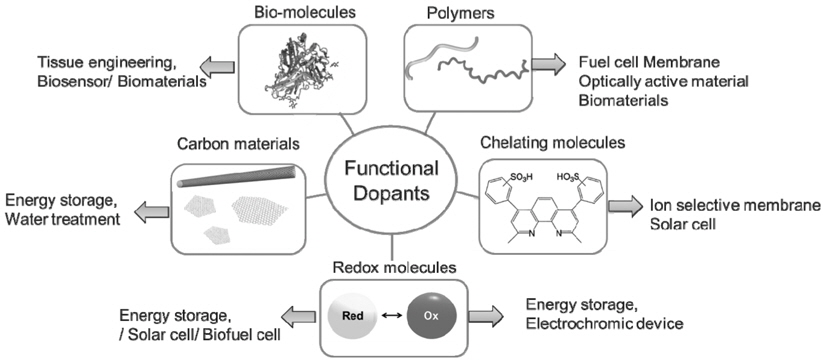
In this article, we focus on CPs doped with functional dopants and their applications. We define “functional dopants” as molecules having specific properties that are introduced to CPs when they are incorporated as dopants during polymerization. For example, pPy doped with ABTS (ABTS = 2,2'-azinobis (3-ethylbenzothiazoline-6-sulfonate)) is redox-active at 570 mV and - 400 mV at pH 1 (All potentials are reported versus Ag/AgCl in this article). The first redox activity stems from the dopant ABTS. Therefore, ABTS is considered as the functional dopant. Electrostimulation of pPy doped with DBS (dodecylbenzenesulphonate) results in volume change larger than that observed with other dopants[10]. The volume change, however, does not originate from properties of DBS. Therefore, DBS is not functional dopants, based on our definition. Categories of functional dopants discussed in this review are: redox-active, electrochromic, biomolecular, anchoring, conductive and chelating. Figure 2 shows types of different functional dopants and their typical applications introduced in this review.
Redox-active dopants include ferrocyanide {Fe(CN6)4-}[11,12], metallophthalocyanines (MePc)[13-16], metalloporphyrins (MePp)[17-19], hydroquinonesulfonate (HQS)[20], tris (2,2’-bipyridine)ruthenium(II) {Ru(bpy)32+} complexed with octasulfonatocalix[8] arene (Calx-S88-)[21], indigo carmine (IC)[22,23], ABTS[23], poly-ABTS and liginin[24]. CPs doped with redox-active molecules can be used as (i) electroactive electrodes in batteries; (ii) electrodes containing mediators to facilitate electron transfer between the active site of an enzyme and an electrode in a biofuel cell or between analytes and electrodes in biosensors; (iii) catalysts for oxygen reduction or oxidation of organic molecules; and (iv) electrodes in photovoltaics to generate photoinduced current.
The key requirements for a functional dopant to be used for electrochemical energy storage applications are that they have to be anionic and have reversible redox chemistry. Two different redox active molecules, ABTS and IC were used as a dopant for a polypyrrole battery cathode and an anode respectively[23]. When two electrodes were combined to form a secondary polymer battery, it showed the open circuit potential ~0.5 V because that ABTS showed reversible electrochemistry at 570 mV and IC at 52 mV (vs. Ag/AgCl). The charge capacity of the battery was 54 C/g. Both of the dopants had two sulfonate substituents, so they remained anionic in both their oxidized and reduced forms. Elemental analysis revealed that the doping ratio (i.e. ratio of pyrrole subunit to dopant) was estimated to be 10 to 1, giving one positive charge for every five subunit of pyrrole. The concentration of the dopants in the polypyrrole was more than 30 times higher than their maximum concentration in water, demonstrating that doping of ABTS and IC could overcome their solubility limit. Therefore, larger amount of faradaic charge could be stored in the dopants in addition to the charge stored by the polypyrrole matrix. Moreover, redox reactions of theses dopants in a conducting polymer could be accessed much faster than those in solution, because their surface confinements enable overcoming the limit of the mass transfer of dopants.
Another interesting example of redox-active dopants is lingosulfonate that contains multiple quinone groups whose theoretical electronic charge density of is up to 1787 C/g[24]. Figure 3(a) shows schematic diagram of two electron redox process of quinone groups of lignosulfonate in polypyrrole matrix. The capacitance of lingosulfonate reached 1000 F/g for thin films and the overall capacitance of the films were-450 F/g (Figure 3(b)). Although, the values of capacitances decreased as the thickness and current rate increased (Figure 3(c)) mainly due to the diffusion limitations. However, these capacitance were higher than most of polypyrrole/ carbon composites[24]. Moreover, the lignin derivative is a cheap biopolymer that is commonly derived from wood as a byproduct of paper processing. Using the cheap renewable biopolymer as a dopant of polypyrrole is attractive idea that will lead to the development of low-cost electrodes.
Many redox-active dopants are also electrochromic, including metallophthalocyanines (MePc)[13], metalloporphyrins (MePp)[17], indigo carmine (IC)[25,26], ABTS[27,28], and poly-ABTS. Incorporation of electrochromic dopants into CPs enables potential-driven changes in color in a CP film in addition to that of CP itself[13,17,27]. A single coating capable of exhibiting multiple colors (CMYK; cyan, magenta, yellow, black) has a technological importance because full color display can be achieved in a one-step deposition process.
To investigate the electrochromism of CPs films containing the electrochromic dopants, spectro-electrochemical studies are required. Figure 4 shows cyclic voltammetry of pPy[ABTS] films and their color as a function of potentials[27]. Polypyrrole itself was a polymeric electrochromogen whose gray color changed to yellow upon the reduction at the potential of -0.3 V (cathodic peak at -0.5 V and anodic peak at -0.15 V vs. Ag/AgCl). The corresponding transmission spectra of pPy films showed that the films were absorptive between 400 and 480 nm and transmittive between 480 and 800 nm. Colorless ABTS become greenish blue ABTS radical with the reversible one-electron electrochemical reaction at 0.5 V, which was also confirmed by the increase of characteristic absorption peaks of ABTS radical at 415, 645, and 730 nm. This concept of combining polymeric electrochromogen and molecular electrochromogen can broaden the choice of color that can be demonstrated by conducting polymers. The pPy[ABTS] films was responded slowly upon the potential change: the coloration time and bleaching time ranged from 2~8 seconds. In general, thicker films showed a higher contrast with slower response. These films were electrochemically and electrochromically stable up to 1500 cycles when the potential applied was less than 0.6 V, whereas, it become unstable when the potential increased to 0.8 V. This instability was attributed to the overoxidation of pPy[27]. The instability of pPy could be improved by using poly (3,4-ethylenedioxythiophene) (PEDOT) conducting polymers. It was reported that PEDOT doped with polymeric form of ABTS (poly-ABTS) exhibited an increased stability to potential cycling both because PEDOT was more resistant to over-oxidation than pPy and poly-ABTS was a non-leachable dopant[29].
2.3. Biomolecular/Anchoring dopants
CPs have been doped with several biological molecules including heparin[30,31], dextran sulfate[32], hyaluronic acid[33], chitosan[34], collagen[35], growth factors[32,36], oligodeoxyguanylic acids[37], ATP[38], a silk-like polymer consisting of fibronectin fragments[39], and the nonapeptide CDPGYIGSR, which corresponds to an amino-acid sequence in laminin that is known to mediate cell attachment and migration and to enhance neurite extension from dorsal root ganglia (DRG)[39].
Unlike CPs in which molecules of interest are embedded as a dopant, polymeric dopants can be used to
The use of dopants to anchor biomolecules onto substrates coated with CP enables several important features. First, confining the bioactive molecules to the surface of CPs ensures that a higher concentration of the bioactive reagent is available to make contact with adhering cells. Second, the concentration of the bioactive molecules remains constant with time because they are attached to the surface of CP via covalent bonds. Third, the surface concentration of the bioactive molecules can be specified and controlled accurately. Figure 5 ((a)-(e)) shows the microscopy images of hippocampal neurons attached to micropatterns of pPy[pGlu]-pLysn, where n is the number of layer [41]. It was clear that the number of neurons and neurite extension on the pattern was directly proportional to the number of the biomolecule layers (n) (Figure 5(f)). Fourth, multi-layers of the bioactive molecules can be fabricated by sequential reactions, which may lead to three-dimensional structures for supporting cells.
Functionalized carbon nanotubes (CNT) can be used as a dopant in CPs[42,43]. The tips or walls of CNTs can be modified with functional groups using a variety of methods including chemical oxidation[43,44], covalent bond formation[45], cycloaddition[46], and - stacking of polycyclic molecules[46]. CNTs modified with anions such as carboxylates (CNT-COO-) were incorporated into pPy as dopants[42]. Impedance data showed that incorporation of CNTs into films of pPy resulted in an increase in the charging capacitance and the knee frequency of these materials: 1.0 F cm-2 and 1.0 Hz for pPy[CNT-COO-] while 0.6 F cm-2 and 0.02 Hz for pPy[Cl-]. Higher conductivity and more porous structure are responsible for the increase of these properties[47].
Another application of CNT-doped CPs (although CNT was used not only as a conducting dopant in this example) is glucose sensing. Functionalized CNTs were incorporated into a pPy film while glucose oxidase (GOx) was entrapped physically in the same film. [48] In the glucose sensor including the film of pPy [CNT-COO-](GOx), GOx catalyzes the oxidation of glucose by oxygen to produce gluconolactone and hydrogen peroxide. The anodic current resulting from the electrooxidation of hydrogen peroxide was measured as an estimate of glucose concentration. Sensitivity and selectivity of the device were improved because the CNT dopants enhanced heterogeneous electron transfer between electrodes and hydrogen peroxide: 130 nA for pPy[CNT-COO-](GOx) versus 10 nA for pPy[Cl-](GOx) at +0.9 V in a solution containing 50 mM glucose. Moreover, the device was operational at potential lower than working potentials of films without CNTs such as pPy[Cl-](GOx).
Other conductive materials of interests are graphene oxide (GO) or reduced graphene oxides (rGO). Doping pPy with the rGOs significantly increased selective adsorption of mercury ions taking the advantage of their high surface area (see Figure 6): when compared to the bare pPy films, the rGO doped pPy films showed more than 5 times more adsorption of mercury ions[49]. Their adsorption capacity reached 980 mg/g and the desorption ratio was extremely high up to 92.3%, demonstrating their potential use for waste water treatment. In addition to the selective adsorption characteristics, the composite showed capability of sensing mercury ions via electrochemically reactions of the metal ions.
Permeability of CP membranes to metal ions can be enhanced by applying potential. When a membrane of CP doped with TS (p-toluenesulfonate, a non-functional dopant) is subjected to a reducing potential that is more negative than the cathodic peak potential of the CP, metal ions invade the membrane to neutralize excess negative charge associated with entrapped anionic dopants. Subsequently, when the CP membrane is subjected to an oxidizing potential that is more positive than the anodic peak potential of the CP, expulsion of metal ions from the CP membrane occurs. Repetition of this oxidation-reduction process ultimately transports metal ions across the CP membrane.
To enhance the flux of metal ions or to separate different metal ions, chelating ligands were used as a dopant in pPy[50]. Bathocuproinedisulfonic acid (BCS) and 8-hydroxyquinoline-5-sulfonic acid (HQS’, different from HQS in redox-active dopants) incorporated in pPy via electropolymerization using the aqueous solution containing pyrrole monomer and the chelating dopants. The doping ratio was found to be 9:1 and 6:1, respectively, and the conductivity was 1.13 and 3.4 S/ cm. Among the tested ions of K+, Co2+ and Cu2+, Ni2+ and Zn+2+ Mg+2+, Ca2+, Mn2+, Fe3+, the pPy[HQS] membranes only allowed the transportation of K+, Co2+ and Cu2+ to a significant extent. Compared to a simple anionic dopant (i.e. without chelating dopant), the transportation of these ions was increased more than 5 times. Moreover, the transportation of Cu2+ was increased more than 10 times by changing the dopant (HQS) to BCS. This enhancement of transportation could be explained by the nature of chelating dopants: chelating dopants could coordinate with metal ions, and could form a stable organometallic complex. The strength of the metal-ligand complex contributed to the flux of metal ions through the CP membrane. Therefore, judicious selection of chelating dopants enables the fabrications of various ion-selective membranes, which will be useful for sensors or other environmental applications.
3. Challenges and Future direction
As we've seen in the previous sections, doping CPs with functional dopants impart specific functions to the materials or enhancement of their performance. However, one major challenge regarding these CPs is the decrease of performance over time. Therefore, in this section, we suggest the ways to improve the stability of the CPs doped with functional dopants, which may find its usefulness in the future development of new CPs.
Most of aforementioned functional dopants were used as the sole dopant incorporated into CP matrix. To broaden the functionality or stability of the CPs, it will be preferable to use multiple functional dopants. As long as the dopant of interest is anionic, it can be added into the electrosynthesis solution containing the monomer of CP and other dopants, so that the multiple dopants can be incorporated into CP matrix. For example, both oxydoreductase and its mediator (e.g. ABTS) were incorporated into polypyrrole to exhibit excellent biocatalytic activity towards oxygen reduction for biofuel cell application. The role of mediator was to improve charge transfer between the electrode and the enzyme by shuttling electrons[51]. Compared to laccase and ABTS in solution, the mediator and enzyme co-entrapped inside the CP matrix showed higher reduction current densities.
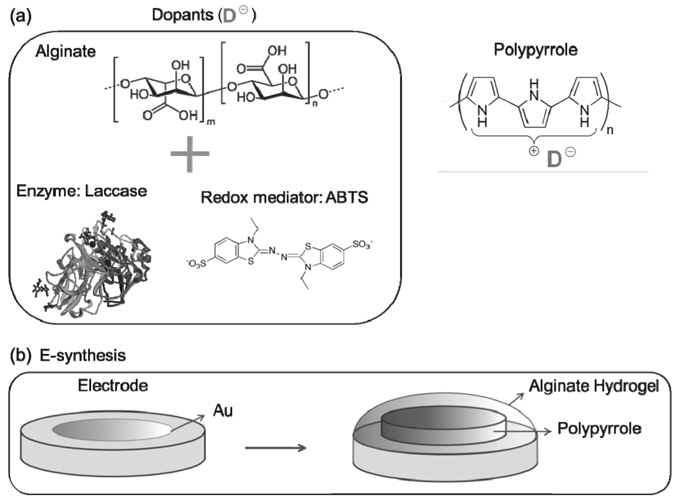
The number of dopants used for biofuel cell electrodes can be further increased to enhance their stability. Alginate is a natural polysaccharide which has been widely used for immobilizing enzyme to prevent fouling and leaching of the enzymes, and therefore improve their overall stability[52]. When alginate was used as a co-dopant of laccase and ABTS (Figure 7(a)), the resulting polymer film showed unique bi-layer structure: an alginate hydrogel layer self-assembled around a pPy inner layer (see Figure 7(b))[53]. Linear sweep voltammetry revealed that the catalytic activity and the stability of the film could be controlled by the concentration of alginate used for the electrosynthesis. At day four, the retention of the catalytic activity was about three times higher for the pPy films with alginate than those without alginate. This improvement in the performance and stability was attributed to the presence of alginate co-doped with mediator and enzyme in pPy film, showing the synergetic effect of multiple dopants for stability and performance.
3.2. Interplays between functional dopants/ electrolytes/ CPs
Many of CPs doped with functional dopants should be investigated thoroughly to improve their stability, because, unlike simple dopants, the physicochemical (or biological) properties of the functional dopants could directly affect the stability of the CP composites. Figure 8(a) shows the factors affecting the stability; the interactions between the functional dopants and CPs; the dopants and electrolytes; electrolytes and CPs. One model CP films presented for addressing the importance of these interactions is pPy[ABTS] films used for electrochromic devices and polymer batteries. As mentioned, these films suffered from instability during their operations. Cyclic voltammetry and UV-Vis spectroscopy study in both aqueous electrolytes (e.g. 0.2 M HCl, LiClO4 in water) and non-aqueous electrolyte (e.g. LiClO4 in acetonitrile) revealed that ABTS leached out from the film only when the aqueous electrolytes were used (Figure 8(b))[41]. This result was found to be due to the ion exchanges in the aqueous electrolyte. ABTS is small (<1 kDa) and anionic in both reduced and oxidized form, so higher concentration of ABTS inside the CP matrix forces ABTS to move out from the matrix and replaced by the anions in the electrolytes. However, in nonaqueous electrolyte, ABTS in the CP films and its electrochemical activity remained constant. Therefore, changing the aqueous electrolytes to non-aqueous one resulted in significant improvement in the stability of the pPy[ABTS] films[41]. Another reason for the instability of the pPy[ABTS] films in aqueous electrolyte was due to the over-oxidation by the dopant (i.e. ABTS)[54]. ABTS has two reversible one-electron transfer redox reactions and undergoes three different oxidized states. In aqueous electrolyte, both the anodic and cathodic peaks (0.75 and 0.4 V) from the first redox reactions were visible, while the cathodic peak from the second redox reaction disappeared (Figure 8(c)). It was found that the potential of the second redox reactions of ABTS was positive enough to overoxidize pPy backbones, resulting in sever loss of the pPy matrix. However, pPy[ABTS] in non-aqueous electrolyte showed two pairs of peaks with no severe decrease of capacity or loss of pPy. The reason for the suppression of pPy overoxidization in non-aqueous electrolyte was explained by the lack of nucleophiles in non-aqueous electrolyte such as water or OH-, whose attack starts the degradation of pPy backbone[55,56]. These examples show that when functional dopants are incorporated, their properties should be thoroughly studied in conjunction with their relationship with CP and electrolyte.
Recent progresses in the developments of CPs doped with functional dopants are presented along with their applications in energy, environmental technology, and nanotechnology. The functional dopants presented in this review include redox-active molecules, electrochromic molecules, carbon nanomaterials, biopolymers, and chelating molecules. Doping CPs with these dopants can either enhance the inherent properties of CPs, or add extra functions to them. The redox active dopants provide extra charge storage capacities or additional electrochromic properties to the CPs. Carbon nanomaterials dopants can enhance surface area of the CPs or selective adsorption of metal ions, which is useful for energy and environmental applications respectively. Bimolecular dopants can be used to improve biocompatibilities of CPs, promoting cell adhesion and growth. Also, by using anchoring biopolymer dopants, concentrations and positions of biomolecules of interest can be easily controlled for use in tissue engineering applications. Chelating dopants in CPs enables controlling ions transportation through the CP membranes. A major challenge of CPs with functional dopants is their instability. This challenge can be circumvented by using multiple dopants, or designing proper combinations of functional dopants, CPs, and environments (e.g. electrolyte) by thorough investigation of their interrelationships.
![(a) Schematic illustration of electrochemical redox reactions of quinone groups in Lig biopolymers that are composited with pPy as dopant. Capacitance as a function of discharge current of pPy[Lig] electrodes with thickness of (b) 0.5 um and (c) 1.9 um.](http://oak.go.kr/repository/journal/15510/CJGSB2_2015_v21n1_12_f003.jpg)
![Coloration of pPy doped with ABTS (pPy[ABTS]) as a function of potentials. Digital photographs of coloration of pPy[ABTS] at each indicated potential.](http://oak.go.kr/repository/journal/15510/CJGSB2_2015_v21n1_12_f004.jpg)
![Schematic illustration of selective adsorption of a toxic metal ion, Hg2+ on pPy[RGO] composite.](http://oak.go.kr/repository/journal/15510/CJGSB2_2015_v21n1_12_f005.jpg)
![Schematic representation of biologically active substrates consisting of pPy doped with pGlu and subsequently modified with multilayers of (A) poly-lysine or (B) laminin. n=number of layers. Immunofluorescent (a-d) and phase contrast (e) images of hippocampal neurons on pPy[pGlu] with multilayers of poly-lysine. (f) Number of cells adhered to patterns.](http://oak.go.kr/repository/journal/15510/CJGSB2_2015_v21n1_12_f006.jpg)
![(a) Schematic diagrams showing factors affecting stability of conducting polymer composite. (b) CVs of pPy[ABTS] at 100 mV/s in aqueous electrolyte. (c) Time-dependant changes of absorption peak of ABTS, when electrodes coated with pPy[ABTS] or PEDOT [ABTS] were stored for 23 h in either aqueous or non-aqueous electrolyte.](http://oak.go.kr/repository/journal/15510/CJGSB2_2015_v21n1_12_f008.jpg)