Anaphylaxis is serious systemic hypersensitive allergic reaction caused by histamine release from mast cells in the cardiovascular system, skin, respiratory tract, and gastrointestinal tract (Johansson et al., 2001; Kim et al., 2013). Because of the potential risk of death, it is critical to recognize anaphylaxis quickly and treat it appropriately. For the management of acute anaphylaxis, an open airway should be maintained. Vascular collapse and angioedema should be treated with epinephrine (Kim et al., 2013; Schummer et al., 2004).
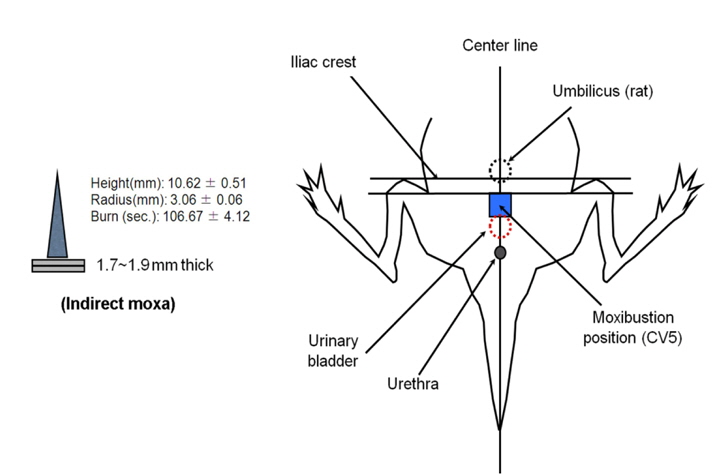
Moxibustion, based on Traditional Oriental Medicine, is a treatment that applies dried
Transient receptor potential vanilloid (TRPV), especially the 1 - 3 subtypes, is thermorecptor that senses physiological stimuli such as heat, protons, osmolarity, and physical stretch (Hu et al., 2004). In addition, in the vascular system, they affect vasoconstriction and vasodilatation (Inoue et al., 2008). TRPV2 was originally isolated as a molecule sensitive to temperatures above 52℃ but has since been shown to be sensitive to some chemicals and mechanical stimuli (Caterina et al., 1999; Hu et al., 2004; Muraki et al., 2003; Shibasaki et al., 2010). TRPV2 has been detected in the brain, autonomic ganglia, spinal cord, skeletal and vascular myocytes, visceral organs (including the intestine, pancreas, spleen, and bladder), and blood cells (Caterina et al., 1999; Lewinter et al., 2008; Muraki et al., 2003). Mechanosensitivity of TRPV2 was first reported in cardiomyocytes (Muraki et al., 2003).
Anaphylaxis causes vasodilatation and hypotension (Johansson et al., 2001). Epinephrine has been described as the drug of choice for the treatment of hypotension from anaphylaxis, but the increased heart rate caused by epinephrine may be poorly tolerated (Weissgerber, 2008). In this study, the effect and specific mechanisms of moxibustion on anaphylaxis animal models and in primary cultured cardiomyocytes were investigated
We purchased compound 48/80, probenecid (TRPV2 agonist), capsazepine, gadolinium chloride (TRPVs blocker), 2-aminoethoxydiphenyl borate (2-APB), ruthenium red, phentolamine methanesulfonate salt, metoprolol tartrate salt, anti-dinitrophenyl (DNP)-IgE, DNP-human serum albumin (HSA), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), dimethyl sulfoxide (DMSO), phosphate-buffered saline (PBS), bovine serum albumin (BSA), ophthaldialdehyde (OPA), Fura-2/AM, epinephrine assay kit, and evans blue from Sigma Chemical Co. (St. Louis, MO, USA); Dulbecco’s modified Eagle’s medium (DMEM), penicillin, streptomycin, and fetal bovine serum (FBS) from Gibco BRL (Grand Island, NY, USA); TRPV2 and actin antibodies from Santa Cruz Biotechnology (Santacruz, CA, USA); Sarcomeric-α actinin antibody from AB cam (Cambridge, England); Fluorescein isothiocyanate (FITC)-conjugated anti-rabbit IgG and tetramethylrhodamine-5-(and-6)-isothiocyanate (TRITC)-conjugated anti-mouse IgG antibodies from Invitrogen (Carlsbad, CA, USA); moxibustion from Gangwhasazabal (Gangwha, Korea).
The original stock of male ICR mice (4 weeks old) and male SD rats (7 weeks old) were purchased from the Dae-Han Experimental Animal Center (Eumsung, Republic of Korea), and the animals were maintained at the College of Korean Medicine, Kyung Hee University. Animal care and experimental procedures were performed under the approval of the animal care committee at Kyung Hee University [Approval no. KHUASP (SE)-13-019]. The animals were housed five to ten per cage in a laminar air-flow room maintained at a temperature of 22 ± 1℃ and a relative humidity of 55 ± 1% throughout the study. No animal was used more than once. The research was conducted in accordance with internationally accepted principles for laboratory animal use and care as found in US guidelines (NIH publication #85-23, revised in 1985).
>
Compound 48/80-induced systemic anaphylaxis
The mice were given an intraperitoneal (i.p.) injection of compound 48/80 (mast cells degranulator, 8 mg/kg) and then moxibustion was applied (moxa, 10.62 mm long and in the diameter of 3 mm; 1, 2, or 3 times stimulation), indirectly to stimulate at the Shimen (CV5) acupoint on the ventral midline of the mouse and rat for 0, 5, and 8 min. The mice were kept in a holder for the duration of the stimulation time. Mortality was monitored for 30 min after induction of anaphylactic shock. 2-APB (60 mg/kg, i.p.) and probenecid (30, 100, and 300 mg/kg, i.p.) were administered after compound 48/80. Capsazepine (10 mg/kg), gadolinium chloride (10 mg/kg), ruthenium red (50 mg/kg), and adrenergic receptor blockers [mixture of phentolamine 10 mg/kg (nonselective alpha-adrenergic receptor antagonist) and metoprolol 20 mg/kg (selective β1 receptor blocker)] were intraperitoneally administered for 30 min before compound 48/80 or probenecid (300 mg/kg) treatment. To assess the thermal effect on anaphylaxis, a soldering instrument at the same temperature as moxibustion was used for treatment.
>
Passive cutaneous anaphylaxis (PCA) reaction
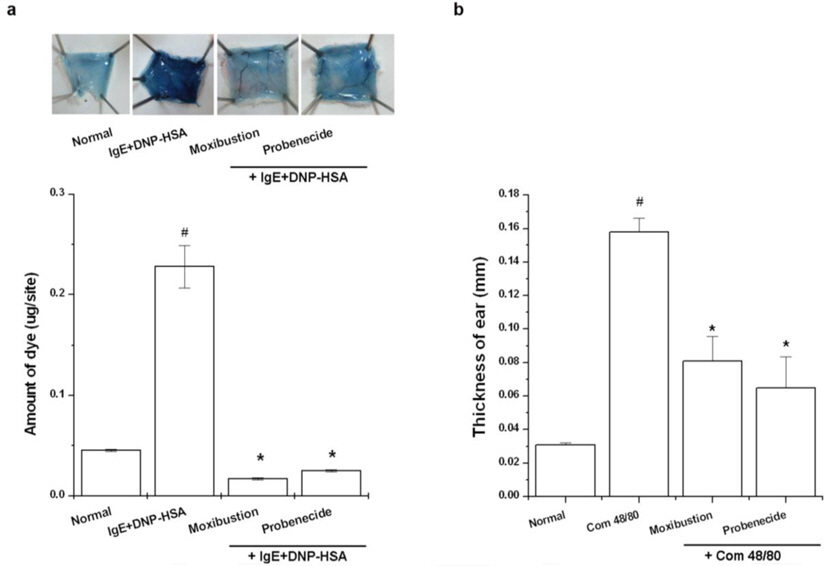
An IgE-dependent cutaneous reaction was generated by sensitizing the skin with an intradermal injection of anti-DNP IgE followed 48 h later with an injection of DNP-HSA into the mouse tail vein. Moxibustion was applied to the CV5 acupoint after DNP-HSA injection and probenecid was administered to some mice 1 h prior to DNP-HSA injection. The amount of dye was determined colorimetrically. The absorbent intensity of the extraction was measured at 620 nm in a spectrofluorometer, and the amount of dye was calculated with the Evans blue measuring-line.
>
Compound 48/80- induced ear swelling response
In this study, ear thickness was measured with a digimatic micrometer (Mitutoyo, Japan) under mild anesthesia. The ear swelling response was determined through any increment in thickness above the baseline control values. Compound 48/80 (100 μg/site) was freshly dissolved in saline and injected intradermally into the dorsal aspect of the mouse ear using a microsyringe with a 28-gauge hypodermic needle. To assess any protective effects from moxibustion or probenecid, moxibustion was applied to the CV5 acupoint after compound 48/80 treatment and probenecid was administered to some mice 1 h prior to compound 48/80. The values obtained represent the effect of compound 48/80 rather than the effect of saline injection (physical swelling), since the ear swelling response evoked by physiologic saline returned to baseline thickness within 40 min.
Histamine levels in serum were measured using the OPA spectrofluorometric procedure. The fluorescent intensity was measured at 440 nm (excitation at 360 nm) in a spectrofluorometer.
Epinephrine levels in serum were measured according to the manufacturer’s specification using the Epinephrine Assay Kit (Sigma Chemical Co.).
Water was removed for 12 h and the rats were anesthetized with zoletil 50® and rumpun® in a 1:2 ratio solution 1 ml/kg. During anesthesia, one of the external iliac arteries was catheritized by laboratory PolyE polyethylene non-sterile tubing (#598321, Havard Apparatus, USA) and connected to an iWorx 214 (iworx system, USA) with a research grade pressure transducer (Havard Apparatus, USA) using LabScribe2 software (iworx system, USA). Five to thirty min after stabilization, compound 48/80 and probenecid were administered and the blood pressure was recorded. Data gained at 20 Hz.
>
Reverse transcriptase-polymerase chain reaction (RT-PCR) analysis
Using an easy-BLUETM RNA extraction kit (iNtRON Biotech, Republic of Korea), total RNA was isolated from organ tissues according to the manufacturer’s specifications. The concentration of total RNA in the final eluate was determined by spectrophotometry. PCR was performed with the following primers: TRPV2(5’ CTA CAG CAA AGC CGA AAA GG 3’; 5’ ACC GCA TGG TGG TTT TAG AG 3’); atrial natriuretic peptide (ANP) 5’ AGG ATT GGA GCC AGA GTG G 3’; 5’ ATA GAT GAA GGC AGG AAG C 3’); WINK4 (5’ TGC CTT GTC TAT TCC ACG GTC TG 3’; 5’ CAG CTG CAA TTT CTT CTG GGC TG 3’); and sodium chloride cotransporter (NCC) (5’ TGA TGC GGA TGT CAT TGA TGG 3’; 5’ AAT GGC AAG GTC AAG TCG 3’). To verify that equal amounts of RNA were used for the reverse transcription and PCR amplification from different experimental conditions, GAPDH (5’ GGC ATG GAC TGT GGT CAT GA 3’; 5’ TTC ACC ACC ATG GAG AAG GC 3’) was used as a loading control. The products were electrophoresed on a 1.0% agarose gel and visualized by staining with ethidium bromide.
>
Quantitative real-time PCR analysis
Quantitative real-time PCR was performed using a SYBR Green master mix and the detection of mRNA was analyzed using an ABI StepOne real time PCR System (Applied Biosystems, Foster City, CA, USA). Primer sequences for the reference gene GAPDH and the genes of interest were as follows: GAPDH (5’ GTT AGT 8 (ADRα2A)(5’ TGT AGA TAA CAG GGT TCA GCG A 3’; 5’ TTC TTT TTC ACC TAC ACG CTC A 3’); beta-1 adrenergic receptor (ADRβ1) (5’ TGG CTT ACT GGC TTG TCTT G 3’; 5’ TTT CCA CTC GGG TCC TTG 3’); and ANP (5’ AGG ATT GGA GCC AGA GTG G 3’; 5’ ATA GAT GAA GGC AGG AAG C 3’). Typical profile times were used the initial step of 95℃ for 10 min was followed by a second step at 95℃ for 10 s for 40 cycles with a melting curve analysis. The levels of target mRNA were normalized to GAPDH levels and compared to the control. Data were analyzed using the ΔΔCT method.
>
Embryonic cardiomyocytes isolation
Pregnant mice were euthanized by CO2 asphyxiation. The embryos (12 days old) were isolated from the uterus and then transferred into a dish with ice-cold sterile PBS without Ca2+ and Mg2+. The embryos were released from the yolk sacs and then transferred into another dish with the same solution as described above. Every single heart was isolated under a microscope using surgical instruments. The heart was incubated at 4℃ for 20 h and subsequently at 37℃ for 15 min in a solution of 0.05% trypsin and 0.02% EDTA (wt/vol) (Biochrom AG). The tissue-trypsin-EDTA-mix was included in 1 ml of DMEM (DMEM high glucose (4.5 g/l) without L-glutamine with 10% fetal calf serum and 1% each of penicillin and streptomycin (Biochrom AG). To dissociate the heart, the tissue was gently pipetted several times. The mix gained by this procedure contains fibroblasts and cardiomyocytes. The cells within the medium from each heart of the same genotype were transferred into a cell culture flask to seed the fibroblast. The cultures were maintained for 1 h at 37℃ in a humidified atmosphere with 5% CO2. The supernatant containing the 9 cardiomyocytes was gently removed, counted, and then plated out at 1×106/ml cardiomyocytes in a 6-well plate coated with fibronectin.
Heart tissue and cardiomyocyte extracts were prepared using a detergent lysis procedure. Samples were heated at 95℃ for 5 min, and briefly cooled on ice. Following centrifugation at 15,000 rpm for 5 min, 50 μg aliquots were resolved by 10% sodium dodecyl sulfate-polyacrylamide gel electro-phoresis. The resolved proteins were electro-transferred overnight onto nitrocellulose membranes in 25 mM Tris, pH 8.5, 200 mM glycerin and 20% methanol at 25 V. Blots were blocked for at least 2 h with 1 × PBS containing 5% non-fat dry milk and then incubated with the TRPV2 antibodies for 1 h at room temperature. Blots were developed by peroxidase-conjugated secondary antibodies and proteins were visualized by enhanced chemiluminescence procedures (Amersham Biosciences, Piscataway, NJ, USA) according to the manufacturer’s instructions.
>
Confocal laser scanning microscopy
Heart tissues were immediately fixed with 4% formaldehyde and embedded in paraffin. After dewaxing and dehydration, sections were blocked with bovine serum albumin followed by a 60 min incubation with anti–rabbit TRPV2 and anti-mouse sarcomeric-α actinin (cardiomyocytes marker) at a concentration of 1 μg/ml. The secondary antibodies, FITCconjugated anti-rabbit or TRITC-conjugated anti-mouse IgG (invitrogen), were added to the incubation for 30 min. The mounting medium containing 4’, 6-diamidino-2-phenylindole (Vector Laboratories, Burlingame, CA, USA) was used to counterstain DNA. All specimens were examined with a confocal laser-scanning microscope. Sections were coded and randomly analyzed by two blinded observers (Jeong H-J and Nam S-Y).
>
Fluorescent measurements of the intracellular calcium level
To measure the time-dependent levels of intracellular calcium, cardiomyocytes (1 × 105) were pretreated with Fura-2/AM in IMDM containing 10% FBS for 30 min and then harvested. After washing twice with calcium free medium (containing 0.5 mM EGTA, an extracellular calcium chelator), approximately 100 μl of the cell suspension were placed into a 96-well plate and treated with compound 48/80, histamine (100 μM), or probenecid (100 μM). The kinetics of the intracellular calcium levels were recorded every 10 s at 440 nm (excitation at 360 nm) in a spectrofluorometer.
The experiments shown are a summary of the data from at least three experiments. Statistical analyses were performed using SPSS statistical software (SPSS 11.5, Armonk, NY, USA). Treatment effects were analyzed by an independent t-test and one way ANOVA with Tukey’s post hoc test.
>
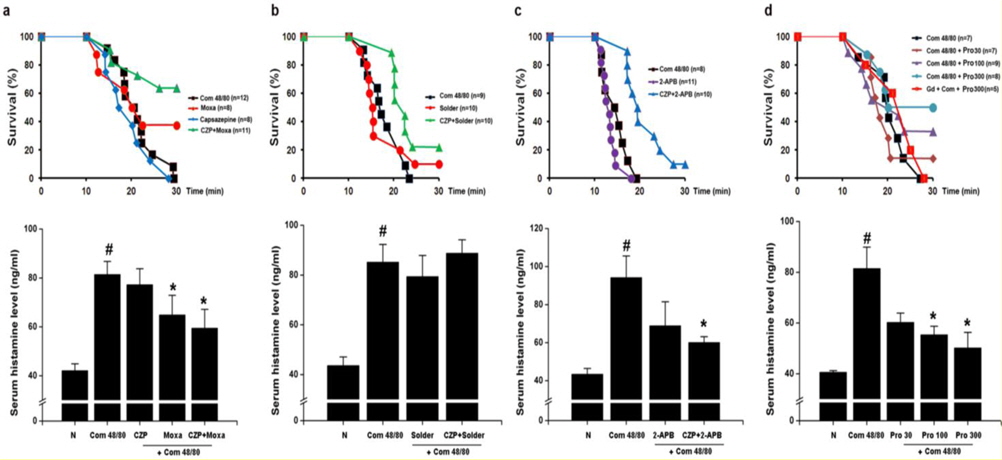
Effect of moxibustion on anaphylaxis
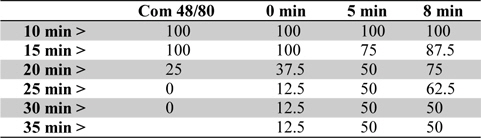
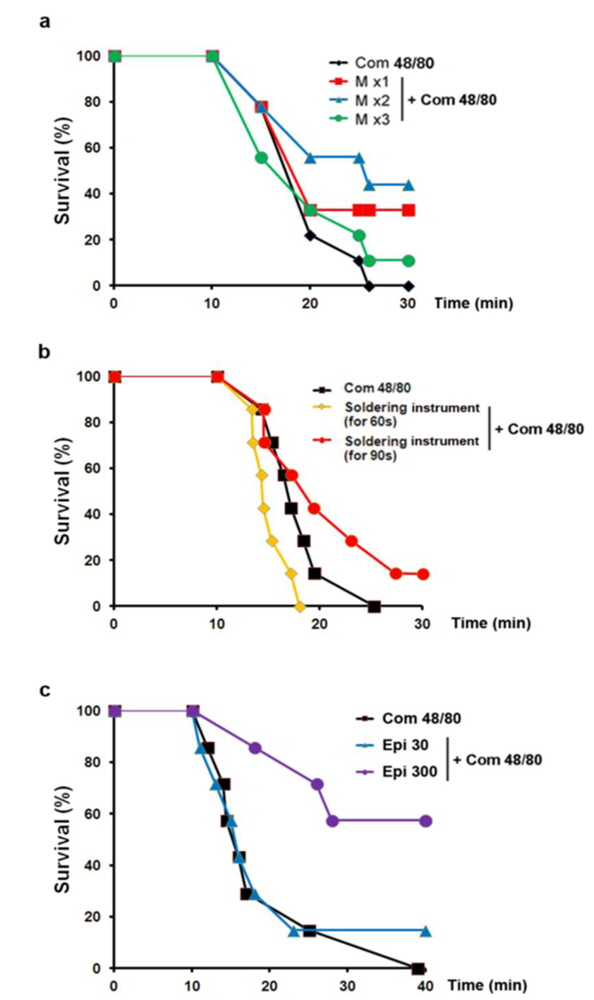
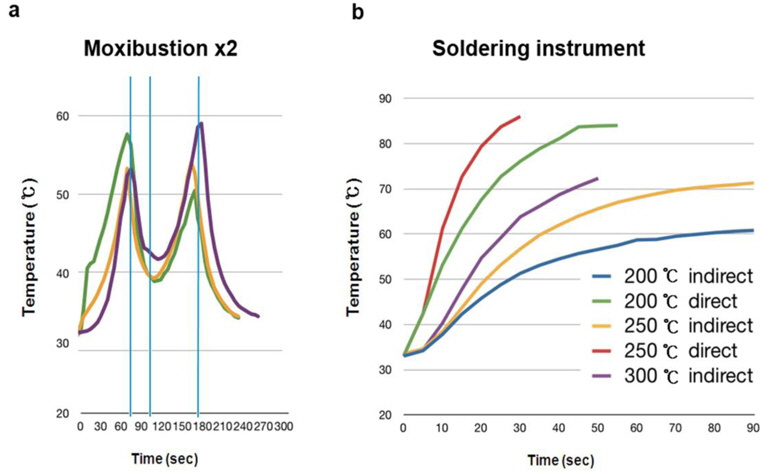
To examine the contribution of moxibustion on anaphylactic reactions, an in vivo model of systemic anaphylactic reaction was applied using compound 48/80 (8 mg/kg) as a systemic fatal anaphylaxis inducer. After the injection of compound 48/80, the mice were monitored for 30 min, and then the mortality rate was determined. Moxibustion was poststimulated 1 (M × 1), 2 (M × 2), and 3 (M × 3) times after 0, 5, and 8 min of compound 48/80 treatment at the Shimen (CV5) acupoint (Fig. 1). As shown in Fig. 1a, an oral administration of saline as a control after compound 48/80 treatment induced a fatal reaction in 100% of mice in each group. However, moxibustion improved overall survival rates compared with the saline control. The most effective moxibustion treatment was two applications at 5 or 8 min after compound 48/80 treatment (Table 1 and Fig. 2). To specifically assess the thermal effect on anaphylaxis, a soldering instrument (200℃ indirect, Fig. 3) was applied at the same temperature as moxibustion. The soldering instrument reduced compound 48/80-induced mortality but moxibustion was more effective than the soldering instrument on anaphylaxis (Fig. 2b). Epinephrine (positive control) reduced compound 48/80-induced mortality (Fig. 2c).
[Table 1.] Survival (%) by time course of moxibustion after compound 48/80 treatment in mice
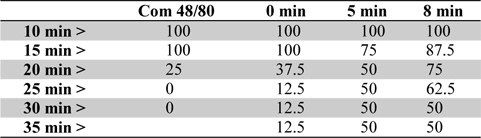
Survival (%) by time course of moxibustion after compound 48/80 treatment in mice
>
Moxibustion activates TRPV2 in anaphylaxis
TRPV1 is well known for its contributions to acute thermal nociception and injury-elicited hyperalgesia (Caterina et al., 2000; Davis et al., 2000). TRPV1 channels are activated by heat, acid, and a large number of chemical stimuli (Hu et al., 2004). To investigate the role of TRPV1 on the inhibitory effects of moxibustion, the effect of capsazepine (a TRPV1 antagonist) on anaphylaxis was examined. Interestingly, capsazepine significantly improved overall survival rates compared with the moxibustion or soldering instrument stimulation groups (Fig. 4a and b, upper). Histamine release was significantly reduced in moxibustion and moxibustion plus capsazepine groups compared with the compound 48/80 group (Fig. 4a, lower panel,
>
Effect of moxibustion and probenecid on allergic animal models
The effects of moxibustion and probenecid were examined in various allergic animal models. Moxibustion and probenecid significantly reduced the PCA reaction, a common murine anaphylaxis model (Fig. 5a,
>
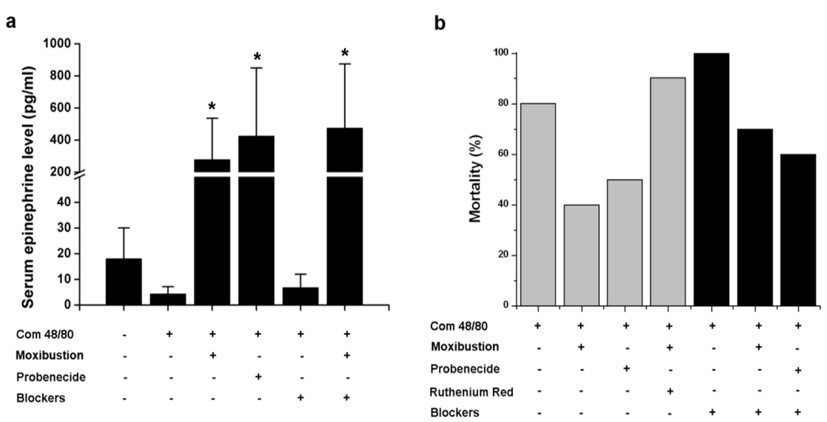
Effect of moxibustion and probenecid on epinephrine secretion
Epinephrine typically used in the treatment for anaphylaxis. Thus, the effect of moxibustion and probenecid were investigated to determine their ability to increase epinephrine secretion in anaphylaxis. As shown in Fig. 6a, epinephrine levels were significantly increased by moxibustion or probenecid (
>
Effect of moxibustion and probenecid on epinephrine secretion
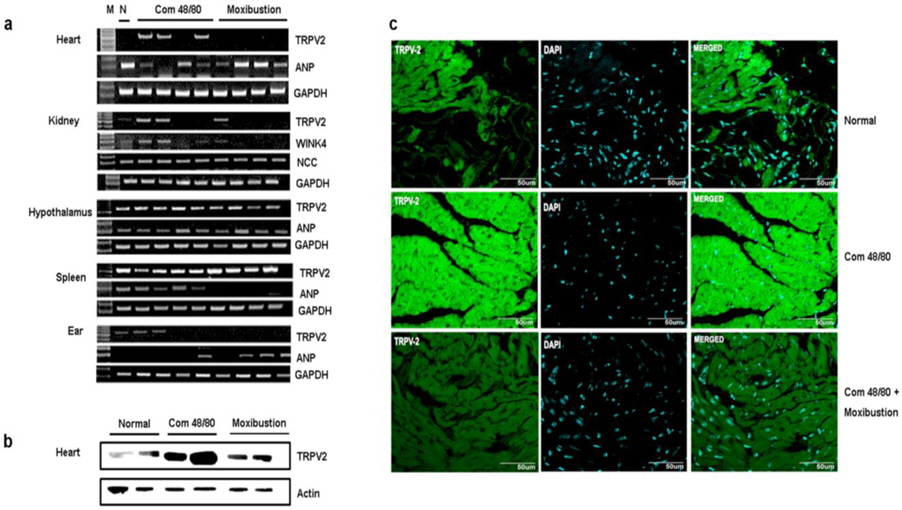
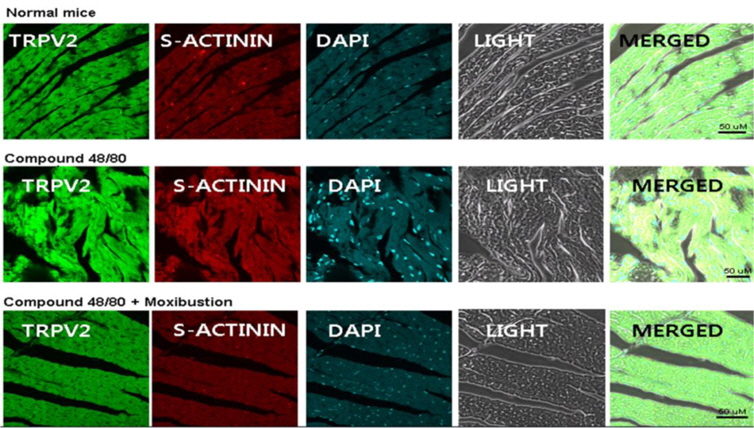
As moxibustion has anti-anaphylactic effect, potentially through activating TRPV2 and by increasing blood pressure through epinephrine, TRPV2 expression and blood pressurerelated factors were investigated in various organs. In previous studies, TRPV2 mRNA was detected in various organs. In this study, TRPV2 expression was investigated in the heart, kidney, spleen, hypothalamus, and ear tissues. The expression of TRPV2 mRNA was only increased through compound 48/80 treatment in heart tissue. Moxibustion decreased the expression of TRPV2 mRNA in heart tissue (Fig. 7a), however, the expression of TRPV2 mRNA by moxibustion in kidney, spleen, and hypothalamus were unchanged compared with the compound 48/80-treated mice. In agreement with the TRPV2 mRNA data, a reduction of TRPV2 protein in the heart of the moxibustion group compared to the compound 48/80 group (Fig. 7b) was observed. Histological analyses also revealed that moxibustion suppressed compound 48/80-induced TRPV2 protein expression in heart tissue (Fig. 7c). In contrast, levels of ANP (a powerful vasodilator) decreased by compound 48/80 were increased by moxibustion in heart tissue (Fig. 7a). Moxibustion had no significant effects on the mRNA expression of WINK4 and NCC (blood pressure-related factors) (Fig. 7a).
>
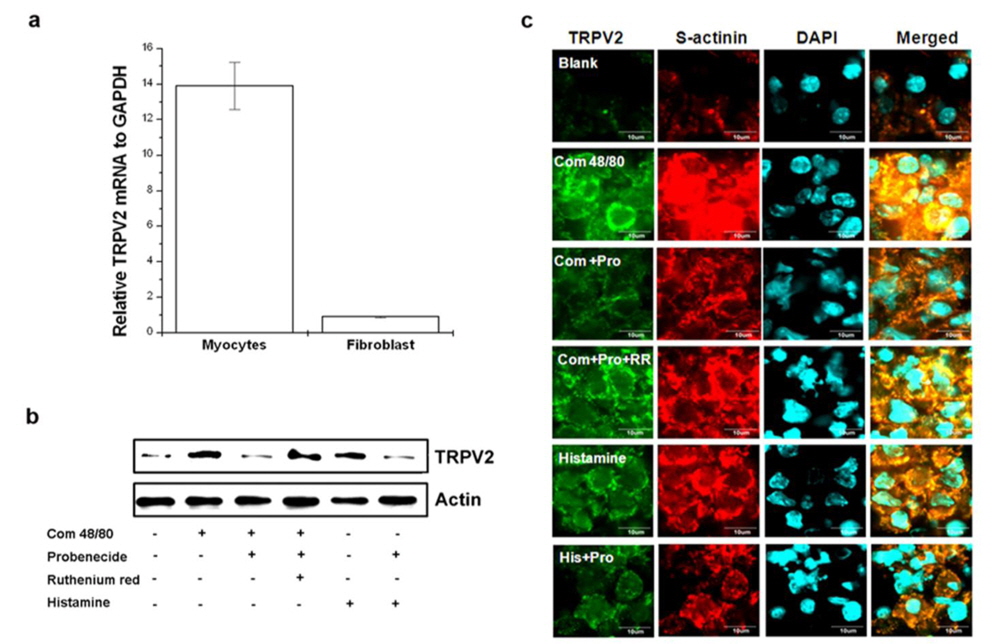
Probenecid suppresses compound 48/80-induced TRPV2 over-expression in cardiomyocytes
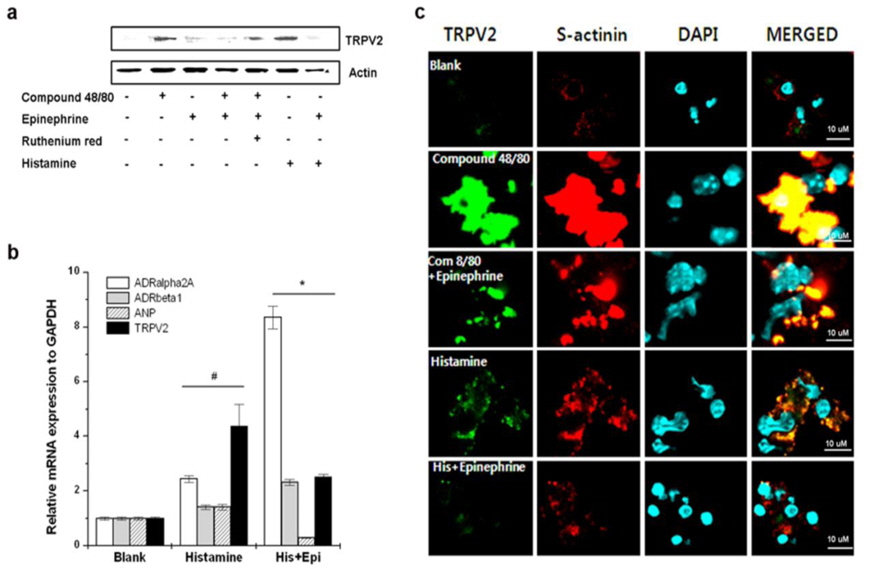
To determine the TRPV2-expressing cells in heart tissue, fibroblast and cardiomyocytes were isolated from heart tissue. TRPV2 mRNA was constitutively expressed in cardiomyocytes but not fibroblasts (Fig. 8a). Compound 48/80 markedly increased TRPV2 protein expression (Fig. 8b). The role of histamine released by compound 48/80 was assessed as well. Histamine also increased TRPV2 expression. However, probenecid decreased compound 48/80 or histamine-induced TRPV2 expression in cardiomyocytes (Fig. 8b). The immunocytochemical analysis of the cardiomyocytes also revealed that TRPV2 was highly expressed in the compound 48/80 group but decreased in probenecid treated group (Fig. 8c). Moxibustion also decreased TRPV2 protein expression in cardiomyocytes of heart tissue (Fig. 9). Epinephrine increases blood pressure (Weissgerber, 2008). Therefore, the effect of epinephrine on compound 48/80-indued TRPV2 over-expression was investigated. Epinephrine significantly decreased compound 48/80- or histamine-induced TRPV2 expression through the modulation of adrenergic receptors (ADRα2A and ADRβ1) in cardiomyocytes (Fig. 10a-c,
>
Probenecid decreases intracellular Ca2+ levels induced by compound 48/80 in cardiomyocytes
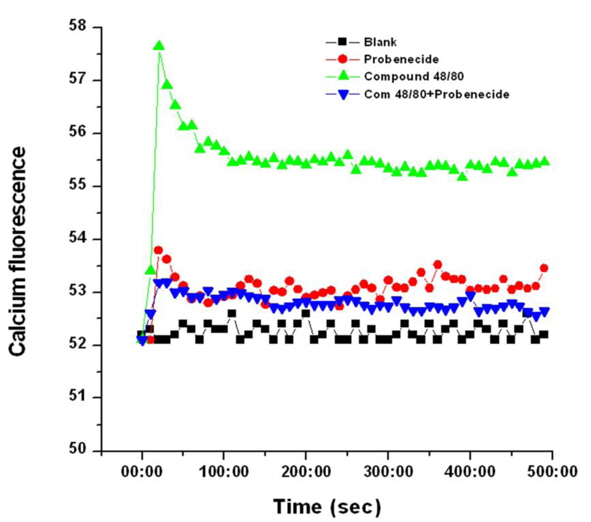
TRPV2 is a moderately calcium-selective cationic channel and increases in intracellular Ca2+ are sufficient for lowering blood pressure (Murata et al., 2004). Thus, the regulatory effect of probenecid on Ca2+ influx in compound 48/80-stimulated cardiomyocytes was examined. Stimulation with compound 48/80 increased Ca2+ release from intracellular stores (in 0.5 mM EGTA containing media), whereas probenecid decreased the level of intracellular Ca2+ increased by compound 48/80 (Fig. 11). Probenecid alone increased the level of intracellular Ca2+.
Anaphylaxis is a severe and potentially life-threatening systemic allergic reaction mostly encountered and managed at emergency departments (Calderón et al., 2013). The most important requirements in the treatment of anaphylaxis are prompt diagnosis and the maintenance of coronary and cerebral perfusion, but these can be difficult or even impossible to accomplish (Schummer et al., 2004). In this study, moxibustion known for its thermal effect attenuated the anaphylactic reaction. Physical stresses, such as heat, can increase sympathetic nerve stimulation and epinephrine release, in turn increasing blood pressure, heart rate, and respiratory rate (Vandana et al., 2013). TRPVs induce vasoconstriction and vasodilatation (Inoue et al., 2008). TRPV1 (> 43℃), TRPV2 (> 52℃), and TRPV3 (< 39℃) are especially activated by thermal stimulation. A previous study indicated that a TRPV1 agonist, capsaicin increases calcium uptake and induces vasoconstriction (Cavanaugh et al., 2011). Capsazepine was therefore used in this study to investigate the role of TRPV1 in moxibustion. However, when capsazepine was treated with moxibustion, the survival rate was higher compared to moxibustion alone. 2-APB can activate TRPV1, TRPV2, and TRPV3 (Hu et al., 2004). When TRPV1-3 are activated by 2 APB, the survival rate was unchanged compared to the compound 48/80 group. In contrast to, the co-treatment of 2- APB and capsazepine reduced compound 48/80-induced mortality. Ruthenium red completely inhibited anti-ear swelling response was significantly reduced by moxibustion and probenecid anaphylactic reaction compared with the moxibustion group (Fig. 5b,
Couto et al. (2013) recently reported that the inhalation of capsaicin is associated with parasympathetic broncho constriction, mucus hypersecretion, vasodilatation, and the sensation of dyspnea. Activated TRPV1 induces mast cell degranulation and releases proinflammatory mediators including histamine (Andrade et al., 2008; Tóth et al., 2009). In this study, capsazepine did not affect compound 48/80-induced mortality by itself. Based upon these results, we hypothesized that TRPV1 and TRPV2 were activated by moxibustion and that the TRPV1 blockade by capsazephine increased survival rate through the modulation of TRPV2 activity. This hypothesis was supported by the ability of probenecid (a TRPV2 agonist) to inhibit the effect of compound 48/80 on anaphylaxis. Therefore, TRPV1 exerted a negative effect and TRPV2 exerted a positive effect on anaphylaxis. This is the first study to show that the anti-anaphylactic effect of moxibustion is potentially associated with the activity of TRPV2.
In this study, the anti-anaphylactic effect of moxibustion was higher than that from the soldering instrument, which did not inhibit histamine release. Therefore, in addition to its thermal effects, moxibustion has various regulatory mechanisms on anaphylactic reaction. Anaphylactic shock decreases blood flow and induces severe arterial hypotension (Johansson et al., 2001). The amount of histamine released from mast cells upon anaphylactic challenge is associated with the reduction of blood pressure and body temperature within the first 10 min of anaphylaxis (Olivera et al., 2010). Epinephrine and vasopressin are drugs that increase blood pressure and are used for the treatment of anaphylactic shock (Kim et al., 2013; Schummer et al., 2004). In this study, moxibustion and probenecid significantly increased the levels of epinephrine. Survival rates increased by moxibustion and probenecid were partially abolished by adrenergic receptor blockers. However, ruthenium red and gadolinium chloride fully inhibited moxibustion and probenecid-induced antianaphylactic effects. The blood pressure in probenecid treated mice was significantly increased compared to mice treated with compound 48/80. Thus, the beneficial effects of moxibustion on anaphylaxis might be derived from the secretion of epinephrine via activation of TRPV2.
TRPV2 is expressed in various organs including the heart, brain, vascular myocytes, and visceral organ. The heart is an important target organ in anaphylaxis and TRPV2 is present and active in cardiomyocytes (Koch et al., 2012). Activation of TRPV2 located within and around the cardiovascular system leads to vasodilatation or vasoconstriction through different pathways (Robbins et al., 2013). In addition, TRPV2-/- mice have depressed myocardial performance (Rubinstein et al., 2014). In the heart, TRPV2 over-expression induces dystrophic cardiomyopathy; however, stimulation under physiologic conditions improves cardiac function (Robbins et al., 2013). Baylie and Brayden reported that TRPV2 contributes to vasoconstriction in vascular smooth muscle. Probenecid has been used to treat for cardiomyopathy (Baylie and Brayden, 2001; Robbins et al., 2013). The cardiac effects by anaphylaxis are also related to the histamine released by mast cell degranulation (Felix et al., 1991). In this study, TRPV2 was over-expressed by compound 48/80 and histamine but this increased TRPV2 expression was decreased by moxibustion and probenecid to a steady-state in cardiomyocytes. Therefore, the regulation of TRPV2 expression could be useful for treating anaphylaxis. Moxibustion may mediate its anti-anaphylactic effects through reduction in TRPV2 over-expression induced by compound 48/80.
Intracellular Ca2+ plays a central role in various biological processes. In the heart, Ca2+ is an important activator of normal muscle contraction. Excessive increases in intracellular Ca2+ induces hypotension, arrhythmias, hypertrophy, apoptosis, and cardiac remodeling (Murata et al., 2004). Therefore, the modulation of Ca2+ homeostasis could be useful for the therapeutic manipulation of these pathophysiological processes. TRPV2 is a Ca2 + selective channel activated by noxious heat (> 52 ℃) and the activation of TRPV2 increases Ca2 + influx in neuronal cells localized to the dorsal root ganglia (Shibasaki et al., 2010). In cardiomyocytes, the stimulatory effects of probenecid on cytosolic Ca2 + and myocyte contractility involve activation of the TRPV2 channel (Koch et al., 2012). Furthermore, the over-expression of TRPV2 in the heart caused cardiac contractile dysfunction due to Ca2 + overloading (Iwata et al., 2003). In this study, compound 48/80 induced TRPV2 over-expression and excessively increased intracellular Ca2+ . These increases were reduced by probenecid. Thus, TRPV2 over-expression by compound 48/80 induces anaphylaxis through increasing intracellular Ca2+ levels in cardiomyocytes.
Traditional Oriental medicine believes that a person is as a whole. The organs and limbs communicate and interact through the meridian system. The meridian system consists of channels and collaterals - pathways for communications between internal and external parts of the body, the flow of qi through blood, and general regulation of the whole body. Moxibustion is related to physiological meridians, cutaneous regions, and acupoints. In this study, moxibustion had an anti-anaphylactic effect through the promotion of epinephrine secretion and the regulation of intracellular Ca2+ by modulating the expression and activation of TRPV2. Therefore, these results provide additional evidence that moxibustion may be an effective new approach for the treatment of anaphylaxis. However, further studies are needed to assess the distinct mechanisms and effects of moxibustion prior to its clinical use in humans.