Prasaplai is a Thai traditional formula that is widely utilized by Thai traditional doctors for relieving primary dysmenorrheal symptoms and adjusting the menstrual cycle (Nualkaew et al., 2004). This formulation first appeared in Vechseuksa, a Thai traditional medicinal text book which was printed in 1908. The formulation comprises each of 8 parts of the pericarp of Citrus hystrix DC., the root of Acorus calamus L., the bulb of Allium sativum L. the bulb of Eleutherine palmifolia (L.) Merr, the fruit P. nigrum L., the fruit of Piper retrofractum Vahl., the rhizomes of Z. officinale Roscoe. and Curcuma zedoaria Roscoe., the seed of Nigella sativa L., and sodium chloride; 1 part of camphor, and 81 parts of Zingiber cassumunar Roxb. All ingredients are separately ground to powder and mixed together. The preparation is mixed with a small volume of water before taken. Historically, Prasaplai preparation was used only for relieving constipation. Later, it was discovered by many traditional practitioners that constipation concurrently occurred with primary dysmenorrhea, and therefore, it had been suggested for the treatment of dysmenorrhea. At present, Prasaplai has also been accepted by the Thai Ministry of Health for the treatment of dysmenorrhea in women after giving birth.
Dysmenorrhea is the medical term for menstrual cramps which causes abdominal or lower back pain just before or during a menstrual period. It affects more than half of menstruating women. One of the major physiological changes that take place in adolescent girls is the onset of menarche, which is often associated with problems of irregular menstruation, excessive bleeding, and dysmenorrhea (Gagua et al., 2013). Dysmenorrhea can be classified into two types: primary and secondary dysmenorrhea. Primary dysmenorrhea results from elevated levels of prostaglandins (PGs), pro-inflammatory cytokines, interleukins, and tumor necrosis factor-α in peripheral blood (Kaplan et al., 2013). Severe myometrial contraction, vasoconstriction, uterine ischemia and pain are resulted from the release of these compounds. Furthermore, progesterone withdrawn before the beginning of menstrual cycle initiates the arachidonic acid release and more cytokine from this acid degradation. Higher cytokine level contributes to higher intensity of dysmenorrheal pain and associated symptoms (Chen et al., 2013).
In western medicines, non-steroidal anti-inflammatory drugs (NSAIDs) are normally administered to treat primary dysmenorrheal symptoms. In spite of their quickness and effectiveness in relieving pain, NSAIDs have many serious side effects on the liver, kidney and gastrointestinal tract. Traditional Chinese Medicine (TCM) is an alternative way to treat primary dysmenorrhea. For example, YuanHu painkiller, which is composed of Corydalis yanhusuo and Angelica dahurica, has been recorded in the 2000, 2005, and 2010 edition of the Chinese Pharmacopoeia (volume one) as a drug for the treatment of primary dysmenorrhea, migrain, and stomachache (Chen et al., 2013).
Prasaplai usage as a preparation to treat primary dysmenorrhea and anti-inflammatory effect was pharmacologically confirmed by Waltenberger et al. (2011). The chemical constituents in each plant component of the preparation concerning cyclooxygenase-1 (COX-1), cyclooxygenase-2 (COX-2) and other inflammatory mediators have been summarized (Waltenberger et al., 2011).
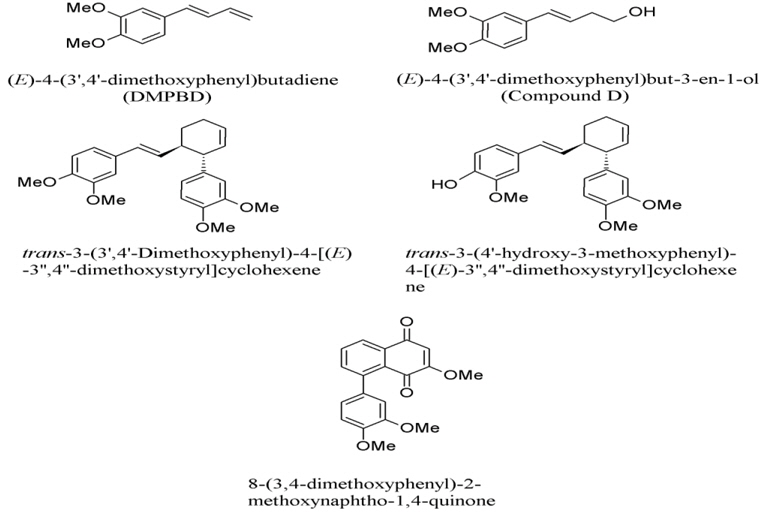
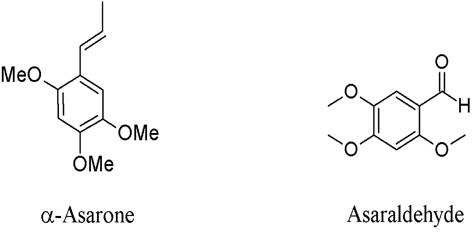
1. Zingiber cassumunar has been reported with IC50 (inhibition concentration at 50%) values against COX-2, ranging from 2.71 to 20.98 μM (Han, 2005). The first two compounds are structurally related to phenyl propane, which are 4-(3,4-dimethoxy-phenyl)but-1,3-diene (DMPBD), 4-[2,4,5-trimethoxy-phenyl)but-1,3-diene (TMPBD), and showed high IC50 of 14.97 and 20.68 μM, respectively (Fig. 1). The other two compounds containing tri-cyclics structure, which are trans-3-(3,4-dimethoxy-phenyl)-4-[(E)-3΄,4΄-dimethoxy-styryl] cyclohex-1-ene and (±)-trans-3-(4-hydroxy-3-methoxy-phenyl)-4-[(E)-3΄,4΄-dimethoxy-styryl]cyclohex-1-ene, exhibited low IC50 of 2.71 and 3.64 μM, respectively. The tri-cyclics structure is selectively fitted into the COX-2 enzyme better than the phenyl propane derivatives (Tangyuenyongwatana et al., 2009). In the lipopolysaccharide-induced expression of cyclooxygenase-2, cis-3-(3΄,4΄-dimethoxyphenyl)-4-[(E)-3΄΄΄, 4΄΄΄-dimethoxy-styryl] cyclohex-1-ene (compound B), cis-3-(2΄,4΄,5΄-trimethoxyphenyl)-4-[(E)-2΄΄΄,4΄΄΄,5΄΄΄-trimethoxy-sty ryl]cyclohex-1-ene (compound C) and DMPBD reduced PGE2 and COX-2 expression in dental pulp inflammation (Aupaphong et al., 2013; Koontongkaew et al., 2013). In addition, curcumin, which was found as a minor compound in Z. cassumunar, has an ability to suppress the expression of COX-2 (Surh and Kundu, 2007). Other mechanisms include the inhibitory effects on lipopolysaccharide (LPS)-induced nitric oxide (NO) production, experimented in mouse peritoneal macrophages in which DMPBD, TMPBD, and cassumunaquinone showed IC50 of 69, 83, and 47 μM, respectively (Nakamura et al., 2009). In addition, Chaiwongsa and co-workers reported an anti-inflammatory activity of compound D against the IL-1 induced catabolic gene expression involved in cartilage degeneration (Chaiwongsa et al., 2013). Compound D behaved as an upstream inhibitor of the catabolic cascade in chronic joint erosion. In regards to immunostimulation, compound D, (E)-4-(2΄,4΄,5΄-trime thoxy phenyl)-3-en-1-ol and (E)-4-(3΄,4΄-dimethoxyphenyl)-3-en-1- methoxy-1-ol, showed significant immunostimulant activity on phagocytosis potential and percentage activity of the phagocytosis (Chairul et al., 2009).
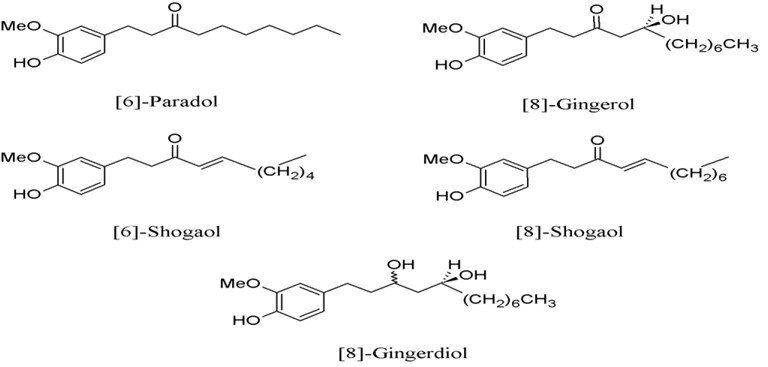
2. Zingiber officinale, a minor component in the Prasaplai preparation, contains five COX-2-inhibitory compounds, which are [6]-shogaol, [8]-gingerdiol, [6]-paradol, [8]-gingerol, and [8]-shogaol (Fig. 2) with IC50 of 2.1, 12.5, 24.5, 10.0, and 7.2 μM, respectively (Tjendraputra et al., 2001).
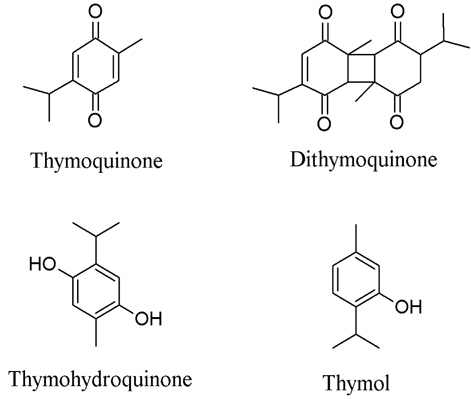
3. Nigella sativa or black cumin is also a minor component in the preparation that shows both COX-1 and COX-2 inhibitory effects. Four compounds, thymoquinone, thymohydroquinone, dithymoquinone, and thymolm (Fig. 3) are responsible for the COX-2 inhibitory activity and exhibited excellent IC50 of 0.3, 0.1, 0.9, and 1.0 μM, respectively (Marsik et al., 2005). Thymoquinone was explored as an anti-inflammatory agent on arthritis in rat models. Signs of inflammation on the claw and radiological signs were searched and tumor necrosis factor - alpha (TNF-α) and IL-1beta were measured. The result showed that thymoquinone suppressed adjuvant-induced arthiritis in rats (Takeoglu et al., 2007).
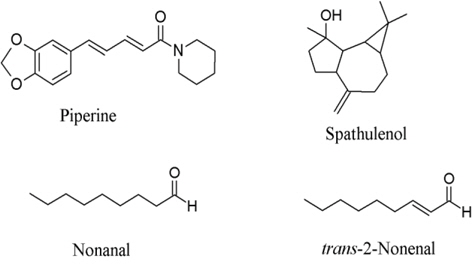
4. Piper nigrum or black pepper is a general household ingredient and is a component in the preparation that showed COX-inhibition activities in the moderate manner. Spathulenol (Fig. 4) inhibited COX-2 receptor 54% at 454 μM (Jayaprakasam et al., 2007) while piperine inhibited prostaglandin synthesis 33.4% at 37 μM (Wagner et al., 1986). Nonanal and trans-2-nonenal reduced arachidonic metabolites by 50% at about 0.25 μM (Sakuma et al., 1997).
5. Acorus calamus is a minor component that contains α–asarone and asaraldehyde (Fig. 5) with COX-1 and COX-2 inhibitory activities. They demonstrated moderate inhibitory activity against COX-2 at 64.39% (480 μM) and 52.69% (510 μM), repectivly.
6. Allium sativum or garlic, of which the ethyl acetate fraction inhibited the LPS-induce dimerization of toll-like receptor 4 (TLR4), resulting in the inhibition of NF-κB activation and the expression of COX-2 and NO synthase (iNOS). The garlic extract could directly inhibit the TLRs-mediated signaling pathway at the receptor level (Youn et al., 2008).
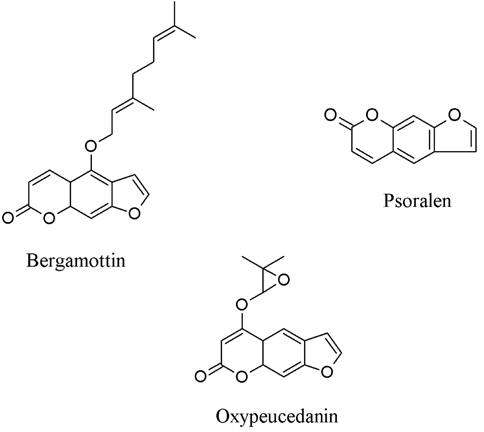
7. Citrus hystrix contains three coumarins, i.e. bergamottin, oxypeucedanin, and psoralen (Fig. 6) which could inhibit both lipopolysacharide and interferon-induced NO generation in RAW 264.7 cells (Murakami et al., 1999).
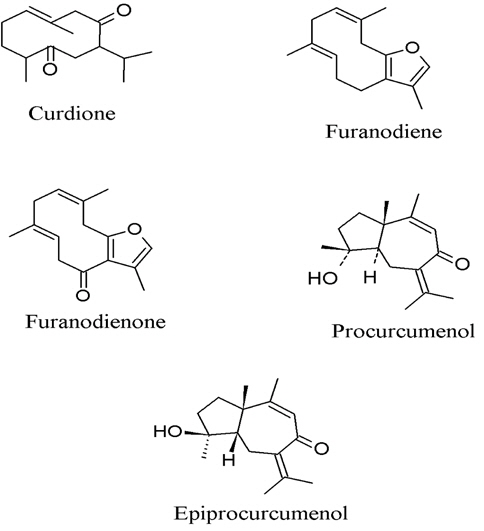
8. Curcuma zedoaria, the anti-inflammatory activity of sesquiterpene furanodiene and furanodienone (Fig. 7) could suppress the 12-O-tetradecanoylphobol-13-acetate (TPA) - induced inflammation of mouse ears by 75% and 53%, respectively, at dose of 1.0 μmol (Makabe et al., 2006). Oh et al. (2007) used bioassay-guided fractionation which led to an isolation of curdione from the rhizome of C. zedoaria with the inhibitory effect on the production of PGE2 in LPS-stimulated mouse macrophage RAW 264.7 cells in a concentration-dependent manner (IC50 = 1.1 μM). Jang et al. (2004) reported active compounds, 1,7-bis(4-hydroxyphenyl)-1,4,6-heptatriene-3-one, procurcumenol, and epiprocurcumenol, isolated from C. zedoaria which inhibited the NO production in LPS-activated macrophages. It was found that 1,7-bis(4-hydroxyphenyl)-1,4,6-heptatriene-3-one was the most potent (IC50 = 8 μM) compound and inhibited NO production through suppression of iNOS expression.
9. Piper retrofractum, the 80% aqueous acetone extract from the fruits of P. retrofractum was found to show protective effects on ethanol- and indomethacin-induced gastric lesions in rats (Morikawa et al., 2004). The extract composes of piperine, piperanine, pipernonaline, dehydropipernonaline, piper loguminine, N-isobutyl-(2E,4E)-octadecadienamide, N-isobutyl-(2E,4E)-eicosatrienamide, methyl piperate and retrofractamide which significantly inhibited ethanol-induced gastric lesions at a dose of 25 mg/kg. All of these compounds may potentially possess the synergistic effect with other herbs.
10. Eleutherine americana, its biological activity of the rhizome was tested on isolated guinea pig heart, and showed effective activity on angina pectoris in a preliminary clinical trial. The naphthalene derivatives, eleuterol, eleutherin, hongconin, and isoeleutherin, were effective in increasing coronary flow of isolated guinea pig heart. When the mixture of naphthalene derivatives was prepared as tablets for clinical use against angina pectoris, it was as effective as dipyidamol in clinical trial (Tang and Eisenbrand, 1992). Paramapojn et al. (2008) reported the anti-acetylchlorinesterase of eleutherin and isoeleutherin with the IC50 of 5.71 and 5.69 μM, respectively. This herb may synergistically enhance other herbs’ activities in the preparation.
Oriental medicines, whether it is TCM or Ayurvedic medicines, often display a similar trend or philosophy in their formulation. Also in Thai traditional medicine, many medicinal plants are selectively and carefully combined for the treatment of diseases. Like TCM, a traditional prescription normally includes different parts, constituting four components - the principal (monarch), adjuvant (minister), auxiliary (assistant), and conductor (guide). The principal herb provides the main curative action; the adjuvant strengthens the principal activity; the auxiliary relieves side effects of the principal herb; while the conductor directs the actions of the principal and adjuvant herbs to the affected area. This preparation philosophy, combining with comprehensive knowledge and wisdom of medicinal herbs, also applies to the Prasaplai preparation. Z. cassumunar, being a major component (50%) in the preparation and possessing potent anti-inflammatory activity, is the principal ingredient. The other herbs, which are Z. officinale, N. sativa, A. calamus, A. sativum, and C. zedoaria, may act as adjuvants on anti-inflammatory activity. P. nigrum and P. retrofractum, having moderate anti-inflammatory activity, may behave as assistant and guide the herbs in the preparation. Piperine from P. nigrum and P. retrofractum, being able to enhance bioavailability of drugs and phytochemical compounds, may enhance the activity of the principal and adjuvant herbs in Prasaplai preparation (Johnson et al., 2011; Lambert et al., 2004).
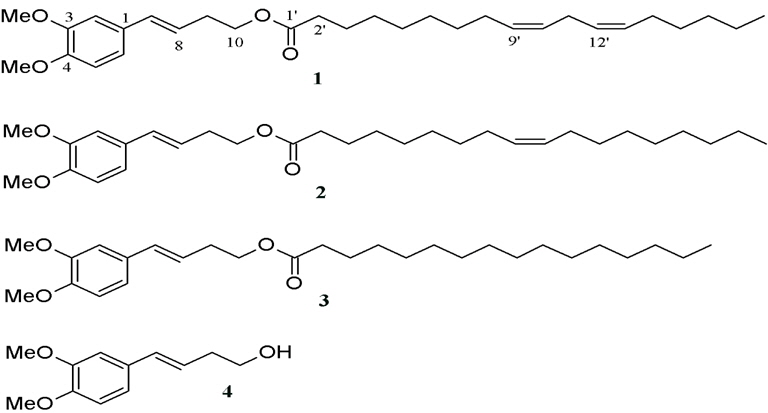
Nualkaew et al. (2004) reported 3 artifacts originated during the storage of Prasaplai. Two artifacts, (E)-4-(3,4-dimethoxyphe-nyl)but-3-en-1-yl linoleate (1) and (E)- 4-(3,4-dimethoxy-phenyl)but-3-en-1-yl oleate (2) were new fatty acid esters. Another one was the known (E)-4-(3,4-dimethoxyphenyl) but-3-en-1-yl palmitate (3) (Fig. 8). The artifacts were found after all the ingredients were mixed together on day 1. After investigating the origin of the artifacts by a systematic preparation of two component mixtures and subsequent High-performance liquid chromatography (HPLC) analysis, they found that the artifacts occurred from the mixture of the rhizomes of Z. cassumunar and the seeds of N. sativa. It was unclear at that time how the esterification reaction initiated in the comparatively dry material of the pulverized drug mixture. However, the authors concluded that this esterification reaction could occur from enzymes catalysis in the solid state environment.
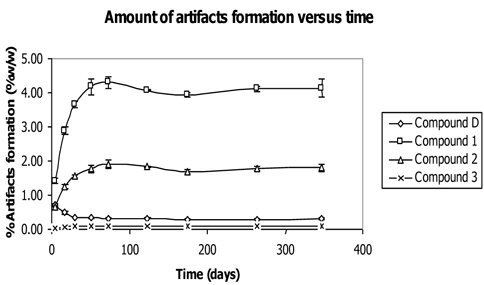
A study was performed on artifacts formation versus time by mixing the powder of Z. cassumunar and N. sativa and keeping the mixture in container protected from light at 25℃ (Tangyuenyongwatana and Gritsanapan, 2010). The mixture was analyzed by HPLC method at certain periods of time within one year as shown in Fig. 9. The result showed the development of each artifact and gave useful information on the artifact formation correlating with time. The amounts of artifacts (compounds 1 - 3) extensively increased in the early period of mixing and continued to amplify in the first 30 days (Fig. 9). Compound 1 was formed at the greatest amount while compound 2 was less and compound 3 showed a slight increment of the amount during the period of time. After 52 days of mixing, the amounts of artifacts formation slowly increased and somewhat plateaued off after. The reaction occurred in the environment of plant material in the dry form which acted as matrix and was capable of absorbing water necessary for the reaction. The capability to hold water of the plant matrix accelerated the reaction to form the products. When the matrix was saturated with water, the formation of artifacts by esterification reaction no longer occurred.
On the other hand, the amount of compound D, ((E)-4-(3,4-Dimethoxyphenyl)but-3-en-1-ol) which was the precursor of all the artifacts, dramatically decreased during the first 30 days after mixing. After that, the amount of compound D slightly decreased, inversely correlating to the amount of artifacts formation.
It was studied the mechanism of the artifacts formation (Tangyuenyongwatana and Gritsanapan, 2008). The study was divided into two experiments. The first experiment was to denature enzymes by heating the rhizomes of Z. cassumunar and the seeds of N. sativa at about 70℃ for 1 h. However, the artifact formation of compounds 1 - 3 still persisted. Consequently, the researchers came up with another proposal: inhibition of the artifact formation with certain amount of water.
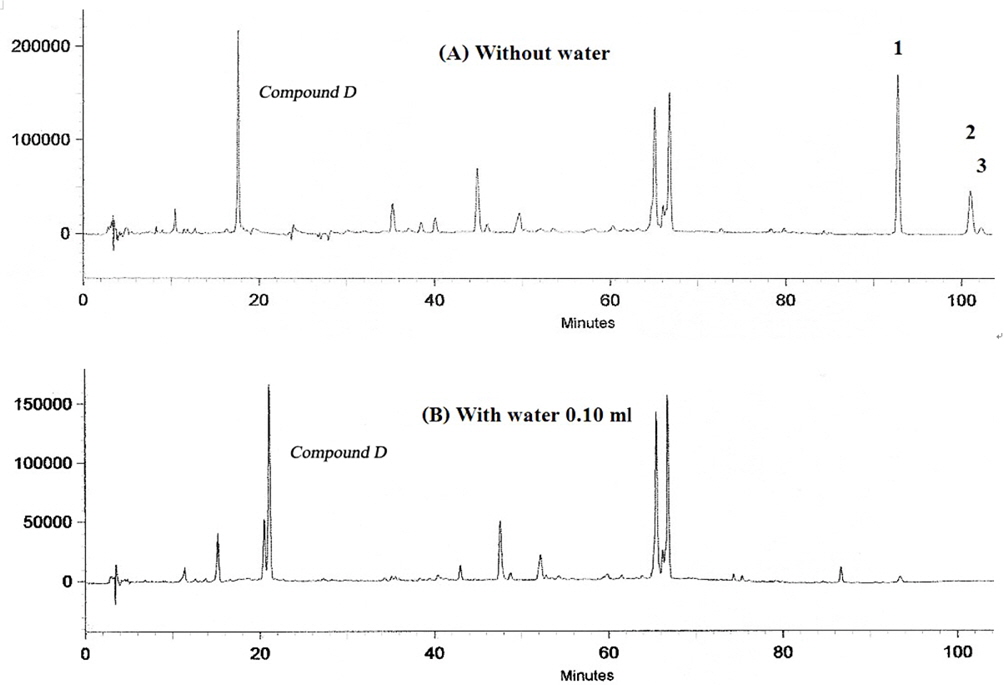
At first, a certain amount of water was added to the mixture of powdered rhizomes of Z. cassumunar and seeds of N. sativa, and they were kept for 20 days. After HPLC analysis, the amount of water that completely inhibited the formation of artifacts was equal to 0.10 ml while the control still showed the artifacts formation (Fig. 10). The addition experiment was based on the solventless chemical reaction between the ingredients in the preparation which we synthesized, (E)-4-(3,4-dimethoxyphenyl)but-3-en-1-ol or compound D as a starting material and directly mixed it with linoleic acid in the presence of anhydrous Na2SO4. Then the mixture was stored in a dessicator for a week and analyzed by HPLC. The artifact (1) still formed at 6% without any enzyme activity, implying that artifacts in Prasaplai preparation occurred naturally after being stored in a dry state at room temperature for a week or more.
The cytotoxicity tests of artifacts against human cancer cell lines NCI-H187, KB, and BC demonstrated IC50 higher than 20 μg/ml for all tests while the positive controls using ellipticine and doxorubicin showed IC50 values at 0.332 and 0.037 μg/ml against NCI-H 187, 0.303, and 0.173 μg/ml against KB, and 0.052 and 0.083 μg/ml against BC cell lines.
For acute toxicity test of each artifact, the dose of 300 mg/kg, did not cause mortality or any sign of abnormalities in tested mice. LD50 values of all artifacts were higher than 300 mg/kg mice (Tangyuenyongwatana and Gritsanapan, 2007). This information ascertains the safety of the artifacts originated in the Prasaplai preparation which has been used as a traditional medicine for a long period of time.
The role of these fatty acid esters in the Prasaplai preparation is an interesting topic in the use of the preparation. One hypothesis is that the fatty acid esters may act as pro-drugs in order to increase the absorption of the parent compound, i.e. compound D. In this in vitro experiment, the artifacts’ absorption of these esters was examined and evaluated their transport across Caco-2 cell monolayers, a standard method for drug permeability test. From the experiment, all esters transported across the Caco-2 cell without enzymatic hydrolysis. The apparent permeability coefficients Papp of compound 1 at 53 μM and 106 μM were 13.94 (0.60) × 10-6 and 14.33 (0.17) × 10-6 cm/s, respectively, while those of compound 2 were 9.45 (0.29) × 10−6 and 10.08 (0.32) × 10−6 cm/s, respectively. Papp values of compound 3 were 7.48 (0.31) × 10-6 cm/s at 53 μM and 8.60 (0.55) × 10−6 cm/s at 106 μM. Papp values of the parent compound (compound D), i.e. (E)-4-(3,4-dimethoxyphenyl)but-3-en-1-ol were 8.53 (0.83) × 10−6cm/s at 53 μM and 16.38 (0.61) × 10−6 cm/s at 106 μM.
Papp of compounds 1 and 2 at 53 μM showed no significant difference compared with those at concentration of 106 μM (compound 1: p = 0.364; compound 2: p = 0.146). Papp of compound D at 106 μM was significantly different from that at 53 μM (p = 0.001). Compound D had Papp values close to those of compounds 2 and 3 at 53 μM, while Papp values of compounds D, 1, 2, and 3 differed significantly (p = 0.001) at 106 μM. The results suggested that the absorption mechanism of compounds 1 and 2 was not dependent on the concentrations of the fatty acid esters. Facilitated transport may be the mechanism. Compound D seemed to follow a concentration dependent passive diffusion mechanism.
The hydrolysis of compounds 1, 2, and 3 did not occur during the transport across Caco-2 cell monolayers. This may be due to the unusually long chain fatty acid structure and other factors such as the duration of the time contact with the enzyme during transport. However, the hydrolysis of the ester linkage in these compounds may occur in the blood circulation or in the liver because these compounds can be hydrolyzed by porcine liver esterase within ten minutes (Tangyuenyongwatana et al., 2009).
Tangyuenyongwatana and Gritsanapan (2009) reported the quantitative analysis of Prasaplai preparation. The recommended dose (1.0 g) of Prasaplai was separately extracted by exhaustive sonication with three different solvents, hexane, 40% ethanol and distilled water. The yields were 26.70 ± 0.11 mg (2.7% w/w), 33.96 ± 0.05 mg (3.40% w/w), and 49.83 ± 0.30 mg (4.98% w/w), respectively. The crude extracts were analyzed by HPLC for contents of the four major compounds, i.e (E)-1-(3,4-dimethoxyphenyl)but-3-en-1-ol (compound D), DMPBD, piperine, ß–asarone, and the three artifacts. The results showed that only the hexane extract contained the artifacts while the 40% ethanol extract contained the highest amounts of the major active anti-inflammatory components; and compound D was found in the water extract only. These results suggest that the 40% ethanol extract should be the appropriate extract for the preparation of Prasaplai in modern dosage forms due to the high content of active antiinflammatory agents in the extract.
For the thin-layer chromatography (TLC) - densitometric method, Tangyuenyongwatana et al. (2012) reported a TLC-densitometric method for the simultaneous quantification of DMPBD in the rhizome extracts of 30 varieties of Z. cassumunar grown in Thailand. This analytical method can also be applied in quantifying DMPBD compound in Prasaplai preparation.
Both analytical methods were validated and are suitable for further research, such as the in vivo studies on bioavailability of compound D, DMPBD, and artifacts. They can also be used to study the content of active compounds of Prasaplai in modern dosage forms like capsule, pellet or tablet.
Kamalashiran and Lekskulchai (2012) performed the comparative study of the efficiency and side effect of Prasaplai extract versus mefenamic acid on relieving primary dysmenorrhea. The study was done on clinical phase II trials. Using 64 volunteers and dividing into 2 groups, the first group (32 volunteers) was given Prasaplai while the second group (27 volunteers) was given mefenamic acid. The clinical trial lasted 3 months and the efficiency of Prasaplai in relieving pain in primary dysmenorrhea showed no significant difference to mefenamic acid during treatment (p < 0.05). Volunteers who took Prasaplai were associated with less pain and side effects compared to the mefenamic group.
Sriyakul et al. (2012) reported a comparative double-blinded randomized phase III clinical trials by using 207 subjects divided into 2 groups. For the control group (n = 104), the patients were given 2 capsules of mefenamic acid (250 mg/cap) which looked like Prasaplai capsules. The treatment group (n = 103) was given 2 capsules (200 mg/cap) of 70% alcohol extract of Prasaplai 3 times a day for 3 days starting at the onset of each menstrual period for 6 periods. In both groups, the severity of pain and other signs of side effects were evaluated and followed up every month. The results showed that there were no statistically significant differences between the two groups in degree of pain relieving. Also, both Prasaplai extract and mefenamic showed no severe side effect.
At the present time, many medical doctors who practice western medicines are interested in oriental medicines, especially Prasaplai. Clinical trials of this preparation have been conducted in many hospitals in Thailand and it is accepted as a potent drug formulation for relieving primary dysmenorrhea.
Prasaplai preparation, which is listed in the Thai traditional common household drug list, is a Thai traditional formula for the treatment of dysmenorrhea and adjustment of menstrual cycle. This preparation was first observed about 100 years ago and is still used until the present time. The active ingredients and biological activities especially on cyclooxygenase mostly come from Z. cassumunar while the other herbs which are A. calamus, A. sativum, C. hystrix, C. zedoaria, N. sativa, P. nigrum, and Z. officinale also possess the anti-inflamatory activity through various pathways. These evidences confirm that Prasaplai preparation has the potential in the treatment of pain and inflammation in dysmenorrhea. In addition, the artifacts occur in the preparation also help the absorption of compound D in the form of fatty acid derivatives which act as pro-drug. The clinical trial showed that Prasaplai was effective for the treatment of dysmenorrhea with similar effectiveness as the standard NSAID drug, Mefenamic acid. In the present time, phase IV clinical trial is being conducted at Mahidol University using Ayurved Siriraj Prasaplai, comparing with Mefenamic acid.