High arsenic (As) groundwaters in deltaic environments are common and wide-spread with documented evidence from many parts of the world (Acharyya et al., 1999; IARC, 1987; Milton, 2005; NRC, 2001; Ratnaike, 2003; Rossman, 2003; Simeonova and Luster, 2000; Wang, 2007). Among the Asaffected deltaic environments, the Bengal Delta Plain is the worst in terms of human exposure (40 million) covering a large geographical area. The groundwater in this region often exceeds WHO permissible limit for arsenic concentration in drinking water i.e. 50 microgram per liter. Arsenic related health problems in the Indo-Gangetic plains have been reported by different research groups (Belon et al., 2007; Chakraborty et al., 2004; Guha Majumdar et al., 1988) but to date there is no remedy. Orthodox medicines such as dimercaptosuccinic acid, diethylenetriamine pentaacetic acid and British Anti Lewisite have been unsuccessful so far to treat these patients, further they have a varying efficacy and harmful side effects, hence there is need for alternative agents which are inexpensive and can easily be procured by common masses. The use of plant and plant materials for curing various ailments have been known to the world since time immemorial and gaining wide acceptance by scientific community, particularly in view of the toxic side effects of most synthetic drugs. Hence the present study is designed to evaluate whether ethanolic leaf extract of
The study was conducted on random bred Swiss albino mice (
Preparation of the leaf extract: The
>
Preliminary phytochemical screening
The presence or absence of phytochemical constituents was analyzed by routine procedures.
Flavonoids (Shinodas test): 100 g of plant material was extracted with 5 ml ethanol and filtered. To 1 (ml) of the filtrate, magnesium ribbon and few drops of concentrated HCl was added. Pinkish red colour indicates presence of flavonoids.
Alkaloids: 25 g of plant material was boiled in 15 ml of 1% concentrated H2SO4 in 50% ethanol and filtered. To the filtrate 5 drops of NH4OH was added followed by 15 ml chloroform and two layers were separated. The chloroform layer was extracted with 15 ml dilute H2SO4. On addition of 5 drops of Mayers reagent to the extract, a creamy red orange, brownish precipitate indicates presence of alkaloids.
Tannins: To 2 ml of the filtrate from the above, 1 ml of ferric chloride was added, a blue to greenish black precipitate indicates presence of tannins.
Carbohydrates: 100 g of
Reducing sugars: 1 ml of above filtrate was boiled with 2 ml of Fehlings reagent for 3 - 4 min. A brick red precipitate indicates presence of reducing sugars.
>
Blood collection and tissue isolation
Blood was collected from retro-orbital plexus and serum was obtained from blood without EDTA by centrifugation for determination of creatinine, bilirubin, catalase, gammaglutamyl transferase and lactate dehydrogenase activity. Blood with EDTA samples was used for determination of G-6PD activity. Liver tissue of sacrificed animal were quickly isolated and separately processed. Briefly 50 mg of liver tissue was homogenized in 10 ml of phosphate buffer and centrifuged at 7000 g for 15 min in cooling centrifuge (C-24BL, REMI, Instruments). Before carrying out the enzymatic estimations the quantitative estimation of total protein was conducted by the method of Lowry et al. (1951).
>
Estimation of mean activities of gama glutamyl tranferase, lactate dehydrogenase
GGT activity was assayed by the method of Szasz (1976). Reagent kit was supplied by Reckon Diagnostics P. Ltd. (Code-6LX010, Baroda, India). LDH activity was assayed by the UV-Kinetic method of Gay et al. (1968). Reagent kit was supplied by Reckon Diagnostics P. Ltd., Gorwa, Baroda, India.
>
Estimation of mean activities of lipid peroxidation (LPO)
The LPO was estimated from the supernatant by the method of Buege and Aust (1984). One milliliter of sample (homogenate containing 0.1 - 0.2 mg of protein) was mixed thoroughly with 2 ml of TCA–TBA–HCl (15% w/v TCA and 0.375% w/v TBA in 0.25-
>
Estimation of mean activities of aspartate aminotransferase (AST), alanine aminotransferase (ALT)
For estimation of AST and ALT in liver tissue samples the methods of Bergmeyer and Brent (1974) was followed with some minor modifications. For AST 0.1 ml of tissue homogenate was made to react with 0.5 ml of the substrate solution L-aspartate and was incubated for 60 min at 37℃. This was followed by addition of 0.5 ml of dinitrophenolhydrazine and then by 5.0 ml 0.4-
>
Estimation of mean activities of acid phosphatase (ACP) and alkaline phosphatase (ALP)
For the study of ACP and ALP, the method of Walter and Schutt (1974) was followed. For ACP, to 2 ml of tissue homogenate 1ml of acid buffer was added, mixed and incubated at 37℃ for 30 min. Then 2 ml of 0.1 N NaOH was added and the absorbance was measured at 405 nm against the standard. For ALP activity 0.05 ml of tissue homogenate was mixed with 2 ml of alkaline buffer and incubated at 37℃ for 30 min; then 10 ml of 0.05 N NaOH was added and the absorbance was measured at 405 nm against the standard.
>
Estimation of catalase activity
Catalase activity in the liver tissue was assayed following the procedure of Sinha (1972). Briefly 0.1 ml of 5% liver tissue homogenate was incubated with 0.5 ml of H2O2 (0.2 M) at 37℃ in presence of 0.01 M phosphate buffer whose pH was 7.4. The reaction was stopped by adding 5% dichromate solution. Then the samples were incubated at 100℃ for 15 min in boiling water. The amount of H2O2 consumed was determined by spectrophotometer at 570 nm and expressed as μmol of H2O2 consumed/min/mg protein.
>
Estimation of superoxide dismutase (SOD) activity
Liver tissue SOD was assayed by the method of Kakkar et al. (1984). Briefly reaction mixture contained 1.2 ml of 0.052 sodium pyrophosphate buffer, 0.1 ml of phenazine methosulfate, 0.3 ml of 300 μM nitroblue tetrazolium. Reaction was initiated by adding 0.2 ml of NADH (780 μM) and stopped by adding 1ml of glacial acetic acid. The colour intensity was determined by at 560nm by UV-1800 Shimadzu spectrophotometer and expressed as units/min/mg protein.
>
Determination of haematological variables
For G-6-PD activity, 500 μl blood was estimated using diagnostic kit procured from Reckon Diagnostic Pvt Ltd., India. The creatinine assay was performed by Jaffe kinetic method. For estimation of total serum bilirubin reagent kit was supplied by Reckon diagnostics (code 64X014). Haemoglobin content was determined by Sahlis method with help of hemometer (Marienfield, Germany).
>
Determination of arsenic from various tissues
Arsenic content in various tissues (liver and kidney), urine and blood was determined by a Perkin Elmer Analyst (AA200) (Texas, USA) Atomic Absorption Spectrophotometer adopting the standard AAS protocol (Belon et al., 2006). 100 μl of each of the urine and blood samples and 1 ml of tissue homogenate was taken separately into 25 ml volumetric flask, to which 5.0 ml of a mixture HNO3/HClO4/H2SO4 (3 : 1 : 1) was added and kept for pre-digestion for about 2 h. Subsequently, the flasks were heated to 150℃ on sand bath. The digestion continued until a colorless liquid 0.5 ml was obtained. All samples were performed in triplicate except blood and urine samples which were performed in duplicate due to less availability of test materials.
Statistical comparisons were made between the positive control + Alcohol groups to that of BV fed group. The significance of difference between data of the different groups was calculated by students’t-test. ANOVA (SPSS 10.0 software) was used to compare multiple groups and within the groups. All the analyses were conducted observer blinded with respect to the animal belonging to treatment group.
Table 1 reveals preliminary phytochemicals which were present during the investigation in the ethanolic leaf extracts of

Preliminary phytochemical screening of Bauhinia variegata leaf extracts which shows presence of several bioactive compounds of which flavonoids and alkaloids are predominant when compared to other compounds
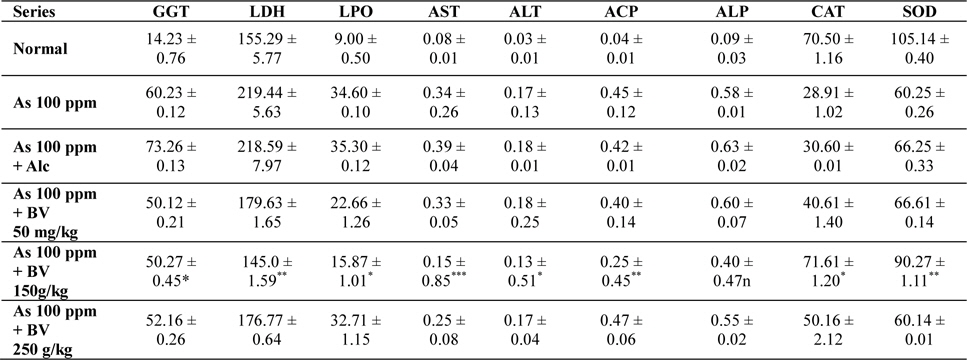
The GGT activity in the As 100 ppm + Ethyl alcohol vehicle of the plant extract (Alc) and As 100 ppm were considerably higher than normal control and As 100 ppm + BV at both the fixation intervals also it increased with the duration of As administration. When the data of GGT activity was compared between As 100 ppm + Alc and As 100 ppm + BV(150 mg/kg), it was appreciably low in BV fed series at both the fixation intervals which was statistically significant (
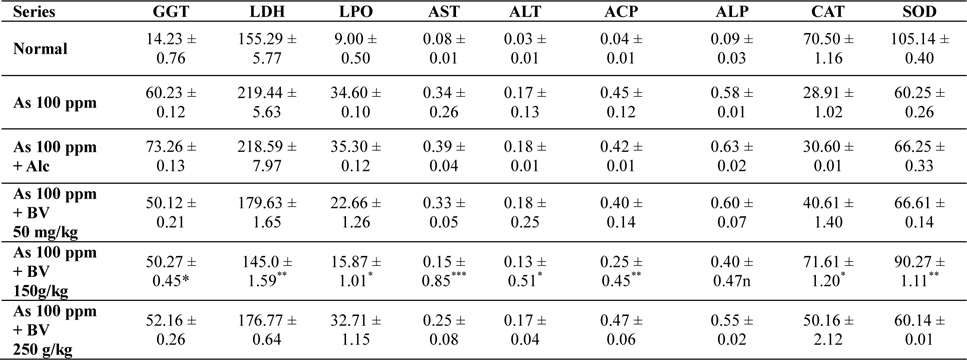
Mean activities of different enzymes in liver of different treated and control series at 15 day fixation interval. GGT-gamma glutamyl transferase, LDH-lactate dehydrogenase activities (IU/L) in serum and LPO-lipid peroxidation (nM MDA/gm of tissue), AST-aspartate transaminase, ALT-alanine aminotransaminase (mM/min/mg in different tissues), ACP-acid phosphatase, ALP-alkaline phosphatase (mM phenol liberated/100 mg protein), CAT-catalase (nm of H2O2 decomposed/min/mg protein), SOD-superoxide dismutase (n moles of CDNB conjugated/min/mg protein)
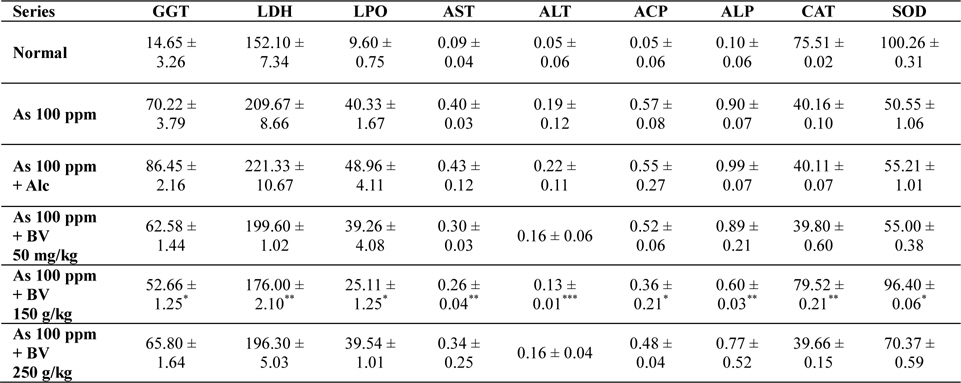
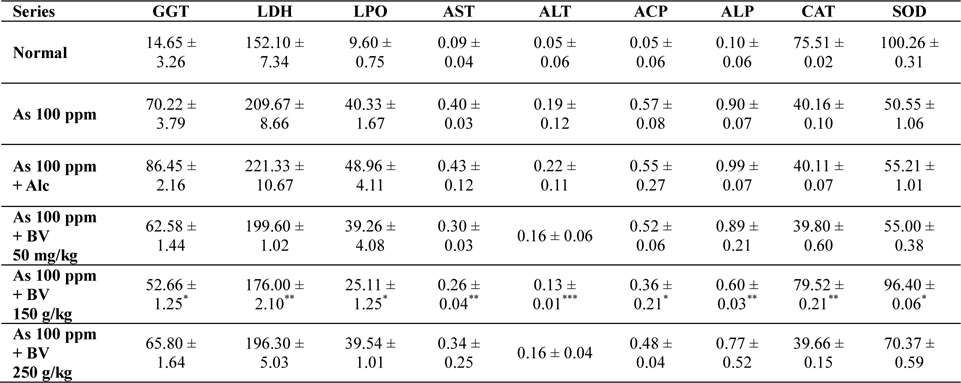
Mean activities of different enzymes in liver of different treated and control series at 30 day fixation interval. GGT-gama glutamyl transferase, LDH-lactate dehydrogenase activities (IU/L) in serum and LPO-lipid peroxidation (nM MDA/gm of tissue), AST-aspartate transaminase, ALT-alanine aminotransaminase (mM/min/mg in different tissues), ACP-acid phosphatase, ALP-alkaline phosphatase (mM phenol liberated/100 mg protein), CAT-catalase (nm of H2O2 decomposed/min/mg protein) , SOD-superoxide dismutase (n moles of CDNB conjugated/min/mg protein)
There was a significant fall in the mean LDH activities in the arsenic 100 ppm + BV (150 mg/kg) fed series at both the fixation intervals when compared to As 100 ppm + Alc and As 100 ppm series.
There was enhanced lipid peroxidation activity in both the fixation intervals in liver tissues of mice fed with As 100 ppm, As 100 ppm + Alc when compared to normal (
On analysis of the results of ACP and ALP there was an increase in activity of both the enzymes in liver tissues in As 100 ppm, As100 ppm + Alc when compared to normal controls (
Oral administration of sodium arsenite decreased both catalase and SOD activity in As 100 ppm, As100 ppm + Alc treated mice at both fixation intervals when compared to normal control and mice fed with As100 ppm + BV which was statistically significant (
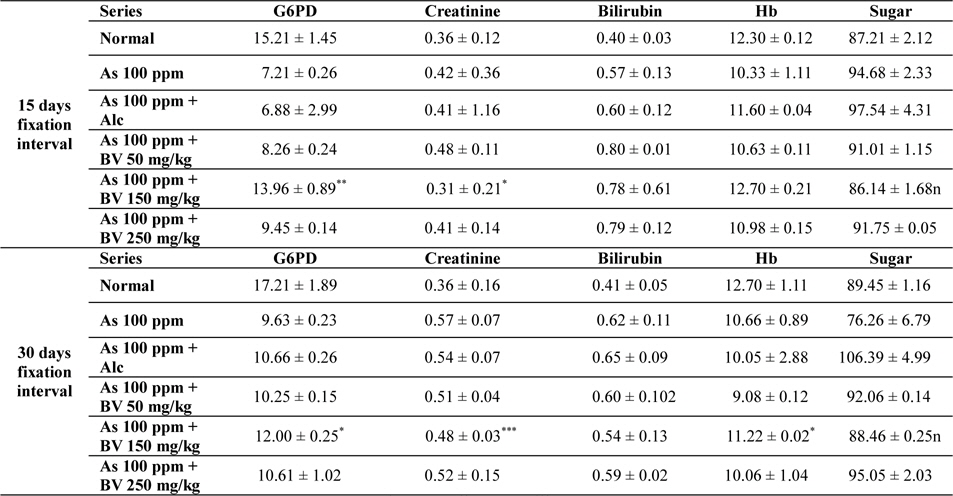
There was a decrease in the mean activities of G6PD in mice fed with As 100 ppm, As100 ppm + Alc at both 15 day and 30 day fixation intervals when compared to normal mice and mice treated with As 100 ppm + BV. The decrease of the activities of G6PD As100 ppm + Alc was statistically significant when compared to As 100 ppm + BV (150 mg/kg) (
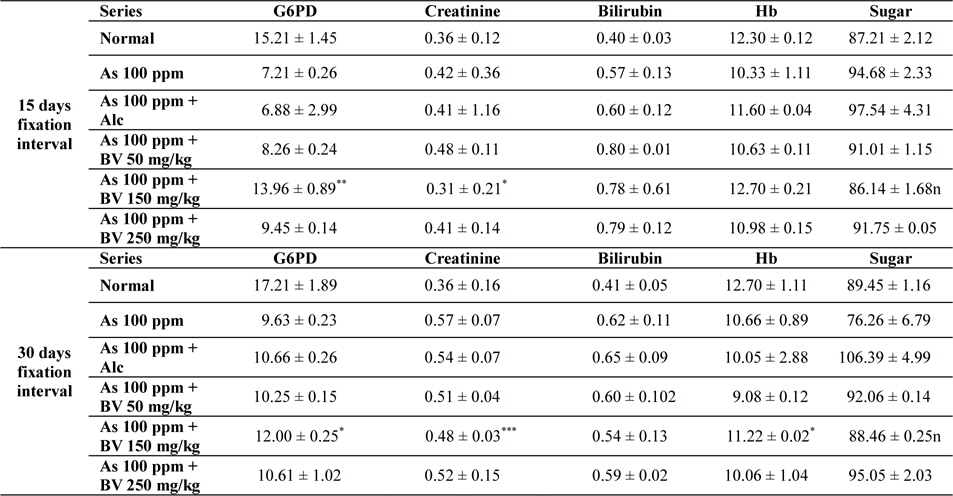
Mean activities of G6PD, serum creatinine content, bilirubin levels (mg/dl), haemoglobin content and blood sugar of mice of different treated and control series at different fixation intervals
In As 100 ppm, As100 ppm + Alc series there was an elevation in serum creatinine, sugar, bilirubin levels and reduction in haemoglobin concentration in As 100 ppm, As100 ppm + Alc when compared to As 100 ppm + BV treated mice (
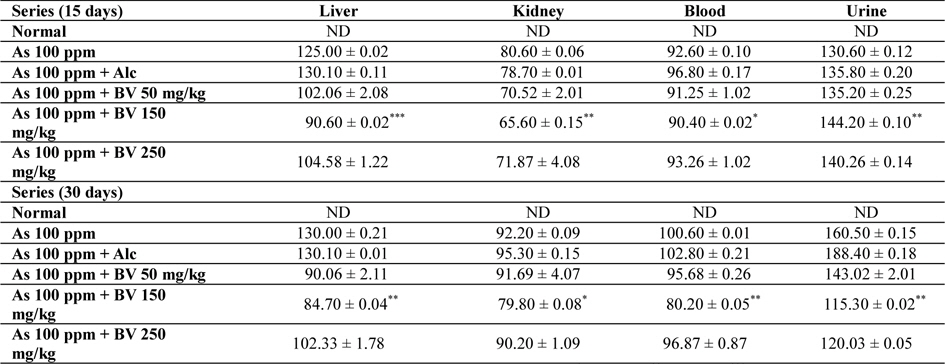
Amount of arsenic deposited in various tissues has been represented in Table 4. The concentration arsenic in liver, kidney and blood in As 100 ppm, As 100 ppm + Alc treated series was appreciably high when compared to normal control at both the fixation intervals. However liberation of arsenic from body via urine was statistically significant in As 100 ppm + BV treated mice when compared to As 100 ppm As 100 ppm + Alc (
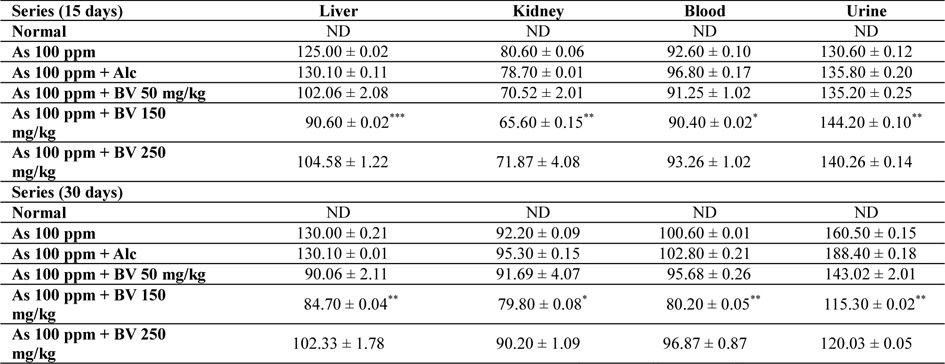
Mean concentrations of arsenic in liver, kidney, blood and urine in different series of mice at 15 and 30 day fixation interval (n = 5) in ppb. (ND-non detected)
The present investigation demonstrates modulation of sodium arsenite induced stress (post arsenic exposure) in mice by administration of BV leaf extract by taking into consideration of various toxicity biomarkers. An analysis of the results of the toxicity biomarkers in As 100 ppm, As100 ppm + Alc fed series at both 15 day and 30 day reveals increase in activities of GGT, LDH, LPO, AST, ALT, ACP and ALP and decrease in activity of G6PD. In As 100 ppm + BV treated mice this trend was largely altered which indicates a detoxifying effect and reduced oxidative stress. This might be due to presence of considerable amounts of phenolic compounds and flavonoids present in the ethanolic extracts of BV leaves which could act as a hydrogen donor antioxidant.
Previous studies reveals that leaves of Bauhinia contains ascorbic acid (Chaturvedi et al., 2011) which might have some role in countering sodium arsenite induced toxicity as revealed in the present investigation. As ascorbic acid has marked nucleophilic properties it might intercept the reactive metabolites which have arisen due to sodium arsenite intoxication thereby preventing their attack and hence have a regulatory role on protein metabolism and repair activities in the cells. Ethanolic extract of the stem of BV showed chemoprevention against N-nitrosodiethylamine in rats and cancer cell lines (Rajkapoor et al., 2006). They investigated that oral administration of BV suppressed various toxicity marker enzymes and also have antitumor activity against Daltons ascetic lymphoma (Rajkapoor et al., 2003). Various other pharmacological studies were conducted with regard to nephroprotective ability of whole stem ethanolic extracts of BV against cisplatin, gentamicin induced nephrotoxicity (Pani et al., 2011; Sharma, 2011).
Chronic feeding of sodium arsenite has been reported to have various toxic effects by generating reactive oxygen species which are directly involved in oxidative damage to DNA, proteins and lipids which ultimately leads to cell death (Garcia-Chavez et al., 2003; Nandi et al., 2005a,b; Sinha et al., 2010). In animals orally administration of inorganic arsenic is methylated to several metabolites mainly dimethylarsinic acid which are excreted from urine and stool (Cohen et al., 2002; Rossman, 2003; Vahter, 1983, Vahter and Concha, 2001; Waalkes, 2007). It is evident in the present study that post arsenic exposure treatment with
However, further in-depth studies are warranted to understand some other aspects of the mechanism of action of the plant extract in showing positive amelioration of sodium arsenite induced toxicity. The doses in the present investigation were selected on the basis of previous studies conducted by us and where we have found that feeding of only
In conclusion this study suggest that administration of an ethanolic extract of