Healthcare-associated infections (HAIs) are a major cause of mortality in the nosocomial environment, endangering lives and increasing hospital costs throughout the world. The incidence of HAIs is variable depending on the country. In the U.S., data show spends between 5.7 and 6.8 billion dollars per year on HAIs (Pittet et al., 2008; WHO, 2011).
The pathogens commonly involved in the etiology of HAIs are bacteria, fungi and viruses; however, bacteria represent the main causal agent (Hughes et al., 2008). The constant and gradual selective pressure caused by indiscriminate use of antibiotics promotes remarkable and rapid microbial resistance. Over the past few decades, an alarming increase in infections caused by antibiotic-resistant pathogens, including methicillinresistant
In order to contribute to the development of new alternatives to control multi-resistant pathogens, different strategies, such as bioprospecting and characterization of new molecules from a wide variety of plants, have been studied. Medicinal plants that are widely used in ethnopharmacology to control disorders and combat infections present vast biotechnological potential, thanks to the presence of multiple bioactive compounds, including antimicrobial proteins/peptides (Benko-Iseppon and Crovella, 2010; Choi et al., 2009; Pascual et al., 2001; Pelegrini et al., 2011; Silva et al., 2011). There is an enormous diversity of antimicrobial proteins, constitutively produced by the host plant or induced by pathological situations, including viral, fungal and bacterial infections, or related situations, including nematodes, insect pests and herbivorous animals (Antoniw et al., 1980; Castro et al., 2005; Iriti et al., 2007; Liu et al., 2006; Tavares et al., 2008). In recent years, screening and isolation of defense plant proteins/peptides with antimicrobial activity has become a new biotechnological tool in the search for new antibiotics (Guaní-Guerra et al., 2010; Lima et al., 2011; Mandal et al., 2011; Maria-Neto et al., 2011; Ribeiro et al, 2010; Silva et al., 2011; Wong et al., 2012).
Antimicrobial proteins from all plant tissues have been widely studied after being isolated from roots, stems, bulbs, leaves, fruits, seeds and flowers; of these, seeds have been used most, due to their higher protein content, compared to other plant parts (Cândido et al., 2011; Lay et al., 2003a,b; Mandal et al., 2012; Maria-Neto et al., 2011; Ribeiro et al., 2010; Sartori et al., 2003). However, flowers may also represent an important source for boosting our antimicrobial arsenal, since this is a structure of extreme value for the species’ survival, responsible for reproduction, and presumed to have great potential in the production of antimicrobial compounds (de Beer and Vivier, 2011; Lay et al., 2003a,b; Moreira et al., 2011; Sartori et al., 2003; Tavares et al., 2008).
In summary, this study aims to expand the knowledge about the antimicrobial potential of proteinaceous extracts from flowers of medicinal and/or ornamental plants from eighteen species that occurs in Brazil. All of them have been traditionally used in ethnomedicine being generally associated with secondary metabolites or essential oils (Favarin et al., 2013; Samy et al., 2013).
Among the medicinal plants selected in this study, some of them are native and/or endemic to Brazil and in most cases are not reported active protein compounds responsible for these activities, such as
Moreover,
The others medicinal plants included in this study are not native, but are widely spread throughout the tropical regions and are easily found in South America, including Brazil, also being known by their medicinal properties including
Lastly, the species
Other species of ornamental plants found in Brazil without records of biological activities tested were also here exploited such as
In resume, experiments here reported were performed using the protein-rich fractions of floral tissues (petals, bracts, inflorescences, spathes, spadix and entire flowers) from different medicinal and ornamental flowering plants, representing numerous botanical families in order to analyze antimicrobial potential toward healthcare-associated bacterial infections. Protein-rich fractions that showed the highest antimicrobial activity were subsequently used for further isolation studies and functional characterization.
>
Biological material and protein extraction
Floral tissues were collected from native Brazilian species and ornamental species acquired in greenhouses, totaling 18 different species and twenty different samples (pink and white variants of
>
Antibacterial activity bioassays
The antibacterial activities of protein-rich fractions and isolated proteins from floral samples were determined by microdilution method (CLSI, 2009). A final concentration of 250 μg/ml from protein-rich fractions from all flower tissues was prepared in sterile 96-well microplates. Subsequent to initial tests, selected samples of 100 μg/ml from DEAE-Sepharose chromatography fractions of H. rosa-sinensis protein-rich fraction were submitted to the same treatment for bacterial growth inhibition bioassays. The wells were filled with 100 μl of Luria-Bertani liquid medium (LB) for antibacterial approach; final bacterial inoculate was 105 CFU/ml and incubated aerobically at 37℃ for 6 h. Sterile distilled water was used as negative control and chloramphenicol 40 μg/ml as positive control. Bacterial growth evolution was monitored by measuring the optical density at 595 nm, every 30 min, to determine the bacterial growth phases. The experiment was performed in triplicate. To perform the bioassays Gram-negative bacteria (Escherichia coli ATCC-8739,
>
Protein isolation of Hibiscus rosa-sinensis L.
After the initial antibacterial bioassays, the H.
>
Molecular mass analyses by SDS-PAGE
Molecular mass profiles from
In recent decades, a significant increase in the resistance of pathogenic bacteria to conventional antibiotics has been observed. Members of the family Enterobacteriaceae, such as
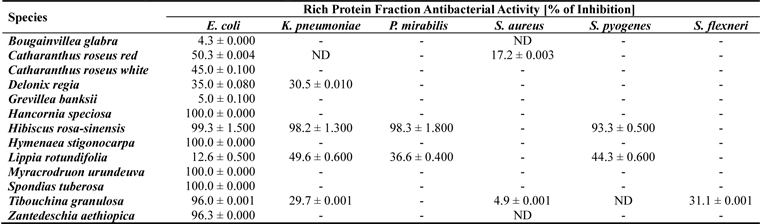
In this study we evaluated the antibacterial activity of floral protein-rich extracts from native Brazilian and ornamental species. The extracts were subjected to bioassays using healthcare-associated infection-related bacteria. Among the 20 samples from 18 flowering plants species studied, only 13 samples presented some bacterial growth inhibition activity. Protein extracts from seven species (
Among the active samples, seven presented remarkable biological activity, showing high or complete bacterial growth inhibitory activity (> 93 - 100%) against most of the microorganisms tested. The human pathogenic bacteria E. coli was inhibited by the extracts from
Due to the higher bacterial growth inhibitory activity against all pathogens tested, added to the cosmopolitan distribution of these ornamental plants, which bloom throughout the year,
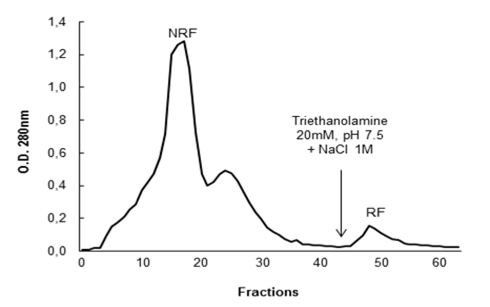
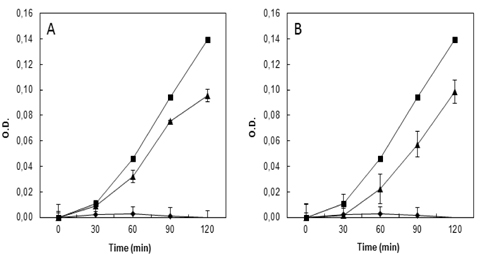
The fractions NRF and RF were dialyzed, lyophilized and then new antibacterial tests were carried out against
[Table 1.] Antibacterial activity of floral protein extracts against bacteria pathogenic human
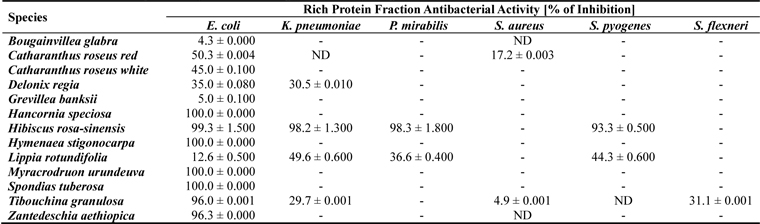
Antibacterial activity of floral protein extracts against bacteria pathogenic human
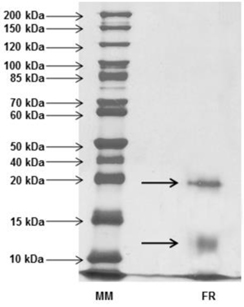
After activity confirmation, the chromatographic fractions were analyzed by SDS-PAGE in order to determine the apparent molecular mass. The protein profiles showed a wide range of molecules with molecular masses lower than 10 kDa to over 100 kDa in NRF, while only two bands were visualized in the RF: one band with apparent mass below 20 kDa and another band with apparent weight below 15 kDa, revealed by silver nitrate staining (Fig. 3). Among all the antimicrobial proteins/peptides isolated from plants, only a few have been isolated from flowers, with representatives in the following families: defensins (Lay et al, 2003a,b; Van der Weerden et al., 2008), snakins (Segura et al., 1999), hevein-type peptides (Koo et al., 1998; Van Damme et al., 1999), lipid transfer proteins (Garcia-Olmedo et al., 1995), myrosinase-binding proteins (Rask et al., 2000), pathogenesis-related proteins (Lotan et al., 1989; Sels et al., 2008), nucleotide binding site and leucine-rich repeat proteins (Moreira et al., 2011).
Analyzing the molecular masses observed in RF, the lower band, between 10 kDa and 15 kDa, may possibly represent classes of antimicrobial proteins, like: Lipid-transfer proteins (LTP), similar to anther-specific sugar beet bvLTP-1 (Beta vulgaris Lipid Transfer Protein 1) with 12 kDa, belonging to a longer group of LTPs. This class has basic proteins with four disulfide bonds and high molecular chains, capable of binding fatty acids and transferring phospholipids between membranes, also reported to be involved in antimicrobial activity and plant defense due to a typical hydrophobic tunnel-like cavity that may be inserted into the microbial membrane, forming a pore and leading to cell death (Capella et al., 2001; Ferreira et al., 2007; Lin et al., 2005; Matsuhira et al., 2007; Tavares et al., 2008). Another possibility is that proteins from the lower band are members of the Myrosinase Binding Proteins (MBP). Actually, two similar forms from
Considering the molecular weight of the RF higher band, less than 20 kDa, due the high molecular mass, it may represent a member of the Pathogenesis Related Proteins (PRP) defense proteins super-family, which has a large variety of molecular sizes and different functions, currently comprising 17 families. One group in which this protein could be classified is the PR-10 family, similar to Parsley “PR-1” with molecular mass up to 19 kDa. This group is formed by Ribosome-Inactivating Proteins (RIP) with RNA N-glycosidase activity that paralyzes protein synthesis from pathogens (Ferreira et al., 2007; Matsuhira et al., 2007). However, an important observation to be discussed at this point is the fact that among the 17 existing classes of PRP, most of them present antifungal activity, which although not tested in this study, permits us to consider a possible classification of the lower protein into, for example, the PR-1 family (with molecular masses about 15 - 17 kDa and homology to the superfamily of cysteine-rich proteins), among a few other cases (Ferreira et al., 2007).
On the other hand, no inferences could be drawn about the NRF, with so many different proteins, as it was impossible to pute the biological activity of the fraction to any observed band, which may contain molecules of any antimicrobial protein class.
Floral protein extracts studied in this research demonstrate the great potential of these species as a source of active molecules in the treatment of bacterial diseases. Although, few antimicrobial proteins or peptides have so far been isolated from flowers compared with other plant tissues, there are promising precedents in the case of NaD1, a defensin isolated from
Until now, there is no record of publications that describe proteins or peptides extracted from flowers that have been found to be active against healthcare-associated infection pathogens, especially bacteria. It is clear that floral tissues have been under-explored in the area of defense mechanisms of reproductive structures, which may be due not only to very low amount of proteins found in this tissue so far, but also to the presence of a variety of pigments and secondary compounds in flowers. The results shown here provide new and important information regarding the purification of proteins with antimicrobial activity, present in native and ornamental flowers, opening new prospects for other studies. Given the great need for implementation of new molecules for pathogenic bacteria control, the authors suggest that these proteins have biotechnological potential for developing new drugs against Gram-negative and Gram-positive bacteria. So, further studies are needed to isolate and identify these possible antimicrobial proteic compounds from the different species here studied and totally purify the antibacterial proteins from