Initial observations made in our laboratories have revealed that repeated daily administration of a medicinally used
Although during more recent years a few reports revealing anti-hyperglycemic activity of diverse types of
>
Plant extract and analytical characterization
The plant material was identified as
Adult Charles Foster rats (150 ± 10 g) of both sexes were obtained from Central Animal House of Institute of Medical Sciences, Banaras Hindu University, Varanasi, India (Registration Number: 542/AB/CPCSEA). They were housed in groups of six in polypropylene cages at an ambient temperature of 25 ± 1℃ and 45 - 55% relative humidity, with a 12:12 h light/dark cycle. Except stated otherwise, they were always provided with commercial Normal Pellet Diet (NPD; Amrut Laboratory Animal Feed; Pranav Agro Industries Ltd., Sangali, India) and water
>
Animal grouping and drug administration
For each series of experiments, six experimental groups consisting of equal ratio of male and female (12 animal each in 2 control groups and 6 animals each in 4 drug treatment groups) were used. Normal and negative control (diabetic and obese) groups were per-orally treated with 0.3% carboxymethylcellulose (CMC) suspension (vehicle) for ten consecutive days. For oral treatments AP was suspended in the vehicle, and 50, 100, and 200 mg/kg/day doses of the extract were administered for 10 consecutive days. Choices of this dose and treatment regimen were based on the observations made earlier in our laboratories with the same extract sample. A positive control group treated similarly either with glibenclamide (Cipla, India; 10 mg/kg/day), or with atorvastatin (Cipla, India; 10 mg/kg/day), was always run in parallel in a given set of experiment.
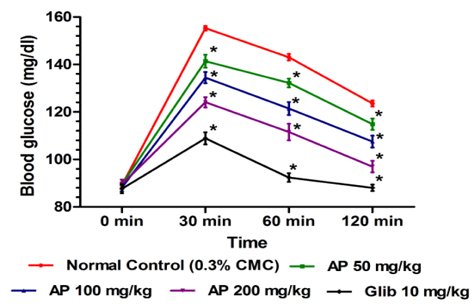
In this test the effects of ten daily oral doses (50, 100, and 200 mg/kg/day) of AP and that of the standard anti-diabetic drug glibenclamide (10 mg/kg/day) were compared. Thirty minutes after the last treatments on day 10 all animals were challenged with an oral dose of 2 g/kg of glucose. Blood glucose levels were measured immediately before glucose challenge and 30, 60 and 120 min thereafter. Hereupon blood was obtained by retro-orbital sampling technique, and an enzymatic method was used to measure glucose concentration (du Vigneaud and Karr, 1925).
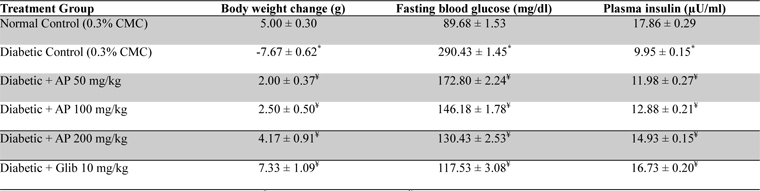
Non Insulin Dependent Diabetes Mellitus (NIDDM/type-2) was induced in overnight fasted animals by a single intra peritoneal (i.p.) injection of 65 mg/kg streptozotocin (STZ; Sigma, India), 15 min after the i.p. administration of 120 mg/kg nicotinamide (SD Fine-Chemical Ltd., India) as described by Masiello et al. (1998) with some modifications. Animal was returned to their cages and provided normal food and 10% sucrose water to minimize hypoglycemic shock. Hyperglycemia was confirmed by elevated glucose level in the blood, determined at 72 hour and then on day 7 after STZ injection (Wu and Huan, 2008). Preselected diabetic animals with blood glucose levels higher than 250 mg/dl were used in the experiments. Body weight changes of experimental animals occurring during the 10-day treatment period were recorded, and thereafter they were fasted overnight for obtaining blood, liver, kidney, and pancreas samples for biochemical and histological analysis using the methods described later. For comparison sake of comparison a group treated with glibenclamide was run in parallel.
Rats were maintained on normal pallet diet (NPD) for one week before the start of the experiment. After one week acclimatization, they were randomly assigned into normal control and obese groups. The normal control group was further maintained on NPD, whereas obese groups were supplied with high fat diet (HFD) for 15 days. Details of the experimental procedure used for obtaining high fat fed obese rats have been described elsewhere (Husain et al., 2011; Srinivasan et al., 2005). Rats showing significant weight gain on HFD (as compared to NPD fed normal control animals) were regrouped for treatments with the vehicle or with AP (50, 100, and 200 mg/kg/day), or with atorvastatin (10 mg/kg/day). Daily treatments were given for 10 consecutive days starting on day 16 of the experiment. Body weights were recorded daily, and after the last treatment day all animals were fasted overnight for obtaining blood samples used for biochemical analysis. Average food intake by each group on day 15 and 25 of the experiment were recorded.
Rats were maintained on normal pellet diet (NPD) and 20% fructose in drinking water for 15 days according to the procedure described elsewhere (Husain et al., 2011a; Jalal et al., 2007). Rats showing significant weight gain compared to normal rats, were divided into different treatment groups. During the subsequent ten day treatment period they continued to receive NPD and 20 % fructose in drinking water till day 25. The vehicle treated normal control group was maintained on NPD and normal drinking water throughout the study period. Body weights of the animals were recorded daily and the volume of drinking water consumed by each group on days 15 and 25 of the experiment were noted. Like in other experiments blood samples for biochemical analysis were obtained after overnight fasting.
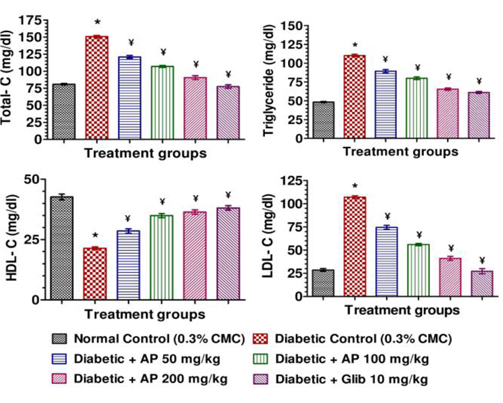
Blood samples were withdrawn from retro-orbital venous plexus on next day of last treatment after appropriate whole night fasting. Plasma was separated from blood in centrifuge at 3000 rpm (845 x g), 5℃ for 5 minute (Compufuge CPR-30, with Rotor No. 8; REMI, India) and plasma (clear supernatant fluid) was kept in freeze till biochemical estimation. Fasting plasma glucose level was estimated by using biochemical enzyme test kit based on GOD-POD method (Span Diagnostic Ltd., India). Plasma total cholesterol, high density lipoprotein-cholesterol (HDL-C), and triglycerides were estimated using biochemical enzyme test kits (Span Diagnostic Ltd., India) (Husain et al., 2011a). Low density lipoprotein-cholesterol (LDL-C) was calculated using Friedewald’s equation (Friedewald et al., 1972). Plasma insulin level was estimated using Enzyme-Linked Immunosorbent Assay (ELISA) test kit (DRG Instruments GmbH, Germany) as described by Husain et al. (2011b). All biochemical analysis were done by using absorbance microplate reader (iMarkTM- Bio-Rad Laboratories, California) according to instruction manual of respective enzyme test kit.
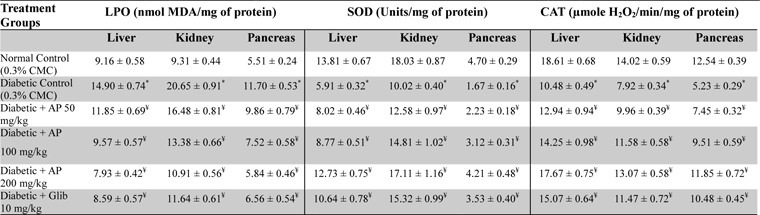
After collection of blood sample, type-2 diabetic rats were sacrificed and liver, kidney, pancreas were isolated to measure their contents in lipid peroxides (Ohkawa et al., 1979), super oxide dismutase (Kakkar et al., 1984), and catalase (Luck, 1973). The experimental procedures used for such purposes are described in details in the references mentioned.
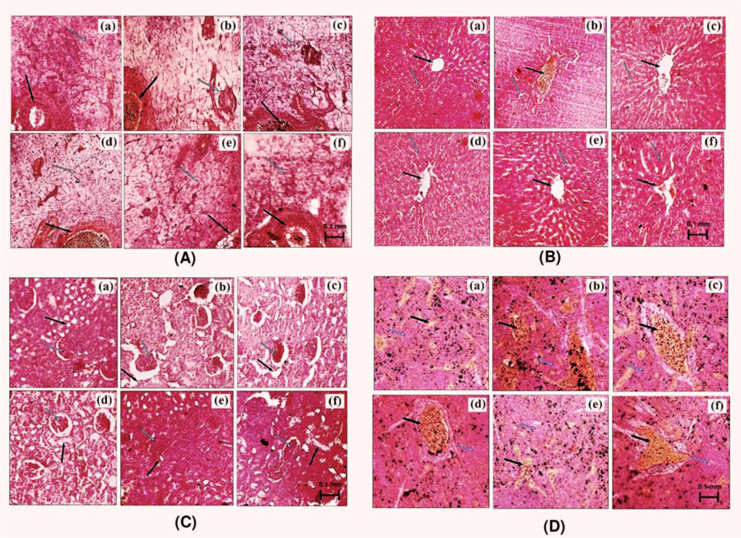
Tissue slices of liver, kidney, pancreas, and spleen obtained from type-2 diabetes animals were fixed in 10% formalin and embedded in paraffin wax. Sections of 5 micron thickness were made using a microtome and stained with haematoxylin-eosin (H&E). For analysis, photographs of each of the slide were taken at 100 X magnification under microscope (Nikon E200-Trinocular Microscope, Japan).
Mean ± SEM was calculated for the observed values in each experimental group. Statistical analysis was performed by one way analysis of variance (ANOVA) followed by Student-Newman-Keuls multiple comparison test (Two-way ANOVA followed by Bonferroni post tests for oral glucose tolerance test). GraphPad Prism 5 was used for statistical analysis. A p-value less than 0.05 were considered as statistically significant.
No statistically significant effects of AP or of glibenclamide treatments on body weight gains of normal laboratory rats during the treatment period were observed (results not shown). Dose-dependent effect of AP was observed 30 min after the oral glucose administration compared to control rats (
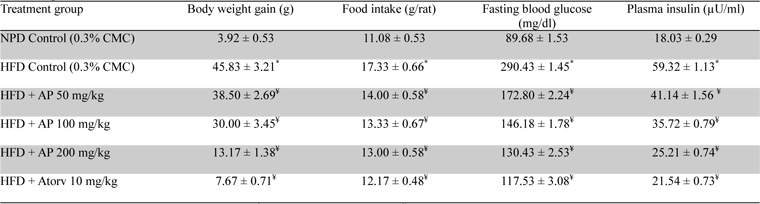
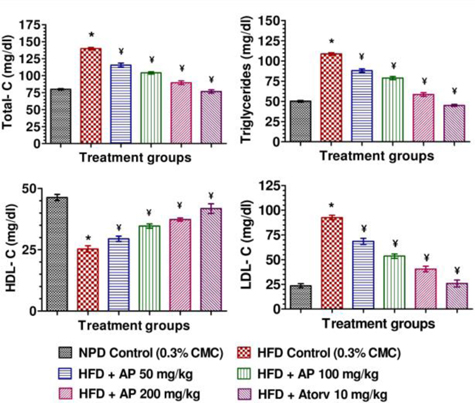
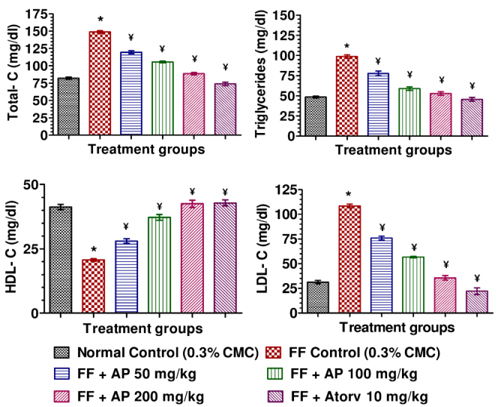
Mean body weights, fasting blood glucose, and plasma insulin levels of the different drug or vehicle treated diabetic groups on the first treatment day were not significantly different from each other (data not shown). During the ten day treatment period the vehicle treated normal control group gained body weight (5.00 ± 0.30 g), whereas vehicle treated diabetic control group lost (7.67 ± 0.62 g). Beneficial effects of AP or of glibenclamide treatments on body weight changes, plasma glucose and insulin levels of diabetic rats are apparent from the data summarized in Table 1. These dose-dependent effects of AP were qualitatively analogous to the anti-diabetic drug glibenclamide. AP treatments dose-dependently reduces the elevated plasma levels of total cholesterol, triglycerides, and LDL, and increases the lowered plasma HDL levels observed in diabetic animals compared to diabetic control (
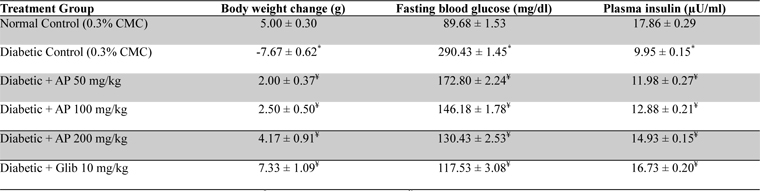
Effect of Andrographis paniculata on body weight, fasting blood glucose and plasma insulin levels of the experimental groups observed 10 days after daily oral treatments
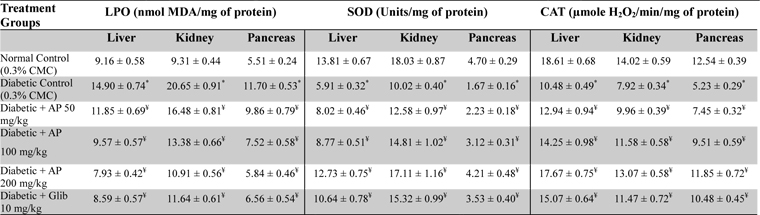
Effects of Andrographis paniculata or of glibenclamide treatments on oxidative status of liver, kidney, and pancreas of diabetic rats
Representative pictures of histological slides of pancreas, liver, kidney and spleen of different groups of animals are shown in Fig. 3. Pancreatic tissues of vehicle treated diabetic rats had diminished glandular acini, islets of Langerhans (necrosis of beta cells), and atrophy in pancreatic duct. These pathologies were less severe in all AP or glibenclamide treated groups (Fig. 3A), and such protective effects of AP treatments were more prominent in the highest dose of AP treated animals. Such were not always the cases for other organ pathologies studied. No beneficial effects of glibenclamide treatment on hepatic pathologies encountered in diabetic animal were observed; whereas these pathologies were less severe, or almost absent, in different AP treated groups (Fig. 3B). Treatments with 50 mg/kg/day AP or with glibenclamide (10 mg/kg/day) had no observable effects on renal pathologies observed in diabetic rats. However these pathologies observed in diabetic rats treated with 100 or 200 mg/kg/day were either less severe or completely absent (Fig. 3C). Although the spleen pathologies observed in the vehicle, or glibenclamide, or 50 or 100 mg/kg/day AP treated animals were qualitatively similar. The histological pictures of the spleens of the 200 mg/kg/day AP treated animals were like those of vehicle treated normal laboratory rats (Fig. 3D).
During the 10-day treatment period the vehicle treated obese rats continued to gain more body weights, and consumed more food and water than the vehicle treated normal control ones (
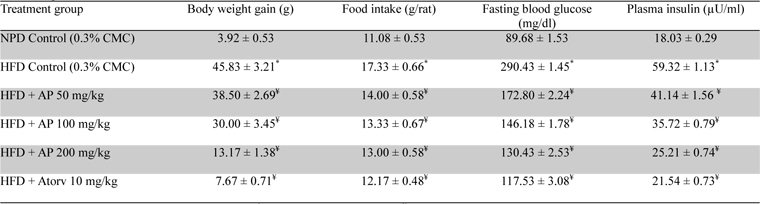
Effects of Andrographis paniculata or of atorvastatin treatments on body weight gain, food intake, and fasting plasma glucose and insulin level in high fat fed obese rats
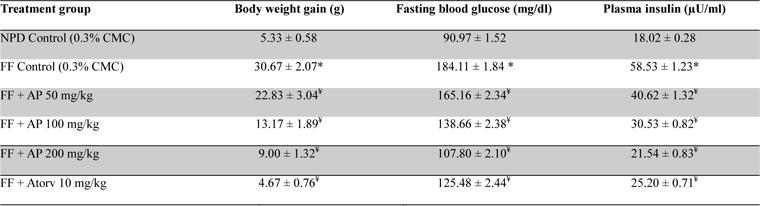
No statistically significant differences between the mean amounts of fructose consumed by the obese rats in different treatment groups were observed (data not shown). Data summarized in Table 4 revealed that the body weight gains of the obese control rats observed during the ten day treatment period were significantly (
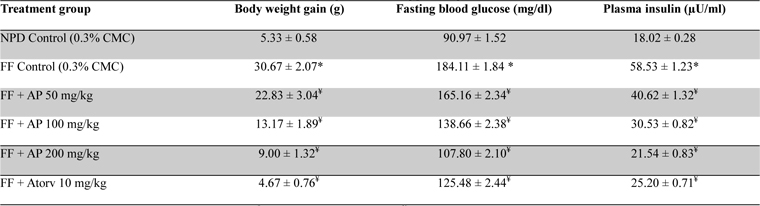
Effect of AP or of atorvastatin treatments on body weight gain and fasting plasma glucose and insulin level in fructose fed obese rats
The analytically well characterized
Observations made in the type-2 diabetes model revealed again that 50 mg/kg/day treatment regimen of AP is effective in reducing all metabolic alterations observed in vehicle treated hyperglycemic and hypo-insulinemic animals and that these observed effects of the extract always increased with its increasing doses. All observed effects of AP on every metabolic parameter assayed were dose-dependent, and were qualitatively analogous to those observed in the glibenclamide treated diabetic animals. However, the histopathological observations made in drug treated diabetic animals were not similar. Although the pancreatic pathologies were less severe in both AP or glibenclamide treated groups, such were not the cases in liver, spleen, and kidney. Unlike in the AP treated groups, no protective effects of glibenclamide treatments against the pathologies of these three later mentioned organs were observed. These observations reaffirm that the mode(s) and site(s) of actions of AP are unlike those of pancreatic insulin stimulating agents like glibenclamide. In any case as previous study (Zhang and Tan, 2000a; Zhang and Tan 2000b; Yu et al., 2003), the observations made in this model clearly reveal that daily oral administration of AP is not only effective in compensating metabolic abnormalities and bodyweight losses in diabetic animals, but also afford protection against free radicals mediated organ damages.
Diabesity associated hyperglycemia and hyperlipidemia are well recognized risk factors of cardiovascular diseases (Braun et al., 2013). Reported observations made in the type-2 diabetes model clearly revealed dose-dependent anti-hyperglycemic and anti-hyperlipidemic effects of AP, and during the course of our studies two reports revealing such effects of very high doses of another type of
Ultimate goal of our efforts to define therapy relevant pharmacological activity profile of AP is to experimentally verify the possibility whether it could as well be an herbal alternative for prevention and treatments of diverse spectrums of mental health problems commonly encountered in obese diabetic patients. Patients with anxiety disorders and depression are more prone to increased dyslipidemia and obesity risk (van Reedt Dortland et al., 2013a), and close association between chronic low grade inflammation and such co-morbidities is well documented (van Reedt Dortland et al., 2013b). Since AP contains andrographolide and other anti-inflammatory molecules, and its anti-stress and other brain function modulating effects of AP has been observed in our earlier studies (Thakur et al., 2013), we are now concentrating our efforts to define its psychopharmacological activity profile in animal models of diabetes and obesity.
Our results revealed that the effects of repeated daily intake of
In conclusion, the results of the present study demonstrate that