Acetaminophen (APAP) is a most common non steroidal analgesic and antipyretic drug used worldwide. APAP exerts few side effects in therapeutic doses, but hepatotoxicity is the frequent consequence of APAP overdose (Olaleye et al., 2010). APAP overdose is responsible for generation of a highly reactive metabolite
Traditional herbal drugs and folk medicinal plants are considered as a key source of new drug molecule or therapy which has attracted considerable attention of researchers. Free radical induced oxidative stress is considered as a key reason for hepatotoxicity, thus a free radical scavenger could be useful to prevent APAP induced liver toxicities (Sen and Chakraborty, 2011). The species Leea possess several biological and antioxidant activities (Saenjum et al., 2007).
>
Plant material and extraction
Leaves of
The fresh leaves were collected and cleaned to remove unwanted materials. The leaves were air dried under shade, pulverized into coarse powder and extracted with methanol using Soxhlet apparatus. The methanol extract was concentrated to dryness under reduced pressure to obtain the methanol extract of
>
Experimental model and treatmentss
Healthy
Thirty rats of either sex were taken and divided into five groups (n = 6) in following manner, ● Group I – Healthy control (saline) ● Group II – Negative control or disease control (saline)● Group III – standard drug (silymarin 25 mg/kg, p.o.)● Group IV – lower dose of methanol extract (extract 150 mg/kg, p.o.)● Group V – higher dose of methanol extract (extract 300 mg/kg, p.o.) Animals were treated with respective test or standard drug or vehicle for consecutive three days once daily. Animals of all groups except healthy control group received single dose of acetaminophen (3000 mg/kg) on 3rd day, thirty minutes after the administration of respective drug treatment (Manokaran et al., 2008; Singh et al., 1995). After 48 h of acetaminophen administration, the blood was collected under light ether anaesthesia and serum was separated by centrifugation of blood at 4000 ×
Biochemical estimation of several serum parameters like serum glutamic oxaloacetate transaminase (SGOT), serum glutamic pyruvic transaminase (SGPT), alkaline phosphatase (ALP), total bilirubin, direct bilirubin, total cholesterol, total triglycerides were carried by using commercial biochemical kit obtained from Agapee Diagnistic Ltd., Kerala.
>
In vivo antioxidant activity
After blood collection animals were sacrificed through cervical dislocation method. The liver of all animal was dissected out and perfused separately with cooled 0.15 M KCl. The liver was centrifuged with 0.15 M KCl - 10 mM potassium phosphate buffer (pH 7.4) to prepare 10% liver homogenate (Kesiova et al., 2006). Liver tissue homogenate was used to estimate the concentration of superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), and reduced gluthathione (GSH).
SOD activity was estimated by the inhibition of autooxidation of epinephrine to adrenochrome at 480 nm in the presence of liver homogenate (Huo et al., 2011; Misra and Frisovich, 1979). Briefly, mixture contain 0.1 ml of liver homogenate, 0.5 ml carbonate buffer (pH 10.2), 0.5 ml EDTA solution. The volume of mixture was adjusted to 2.5 ml. Epinephrine solution (0.5 ml) was mixed to initiate the reaction. Auto oxidation of epinephrine to adrenochrome was carried out in a control tube without the homogenate. The SOD activity was calculated using a molar extinction coefficient of 4.02 × 103 M−1cm−1, and expressed as μM/min/mg protein.
CAT activity was determined by measuring the decomposition of H2O2 to H2O (Huo et al., 2011). Briefly, 0.1 ml of supernatant was mixed with 1.9 ml of 50 mM potassium phosphate buffer (pH 7.0) and the reaction was initiated by the addition of freshly prepared 0.1 ml of 30 mM H2O2. The rate of H2O2 decomposition was determined spectrophotometrically at 240 nm. The result was calculated using a molar extinction coefficient of 43.6 M/cm and expressed as μM H2O2/mg protein/min.
Briefly GPx was estimated by using taking 100 μl tissue homogenate solution and 800 μl 100 mM/l potassium phosphate buffer (pH 7.4) containing 1 mM EDTA, 1 mM sodium azide, 0.2 mM NADPH, 1 U/ml glutathione reductase, 1 mM GSH. After 5 min incubation 100 μl 2.6 mM H2O2 was added and the absorbance change at 340 nm in 3 min was recorded at 37℃ (Hsu et al., 2007). Activity of GPx was calculated using the molar extinction coefficient of NADPH 6220 M−1cm−1 and expressed as μM NADPH oxidized/min/mg protein at 37℃.
GSH content in liver was estimated based on the reaction of GSH with 5,5’-dithiobis (2 nitro benzoic acid) (DTNB) to produce a compound that absorbs at 412 nm (Raphael, 2004). Liver homogenate (0.5 ml) was mixed with 125 μl of 25% TCA. The test tubes containing the mixture were kept on ice for 5 min. Exactly, 0.6 ml of 5% TCA was mixed with the previous mixture and centrifuged at 1500 rpm for 10 minute. After centrifugation, 0.3 ml of supernatant was mixed with 0.7 ml of phosphate buffer (0.2 M, pH 8) and 2.0 ml DTNB solution (0.6 mM in 0.2 M sodium phosphate buffer, pH 8). The absorbance of solution was measured after 10 min 412 nm. The GSH content was determined using a standard curve varying from 5 - 100 nm in 5% TCA for assay, and results were expressed as nmol/mg protein.
GSH content in blood was estimated by the method of Khynriam and Prasad (Khynriam and Prasad, 2001). Blood sample (0.1 ml) was mixed with 0.9 ml of water and 1.5 ml of precipitating solution (1.67 g glacial metaphosphoric acid, 0.2 g sodium EDTA, 30.0 g NaCl in 100 ml water), and the mixture was incubated at room temperature. After 5 min of incubation period, the mixture was centrifuged at 3000 ×
The data were expressed as mean ± SEM (n = 6) and subjected to analysis of variance (ANOVA). Statistical analysis was carried out by analysis of variance followed by Tukey tests, using SPSS (Statistical Package for Social Sciences) version 10.0 software. A level of
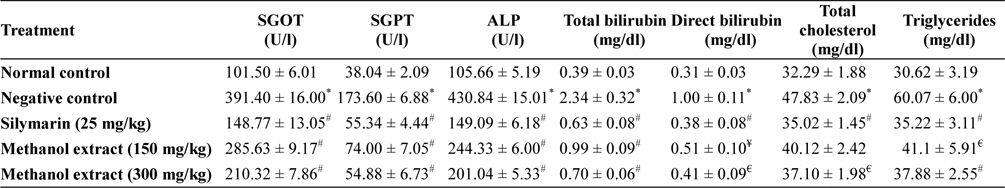
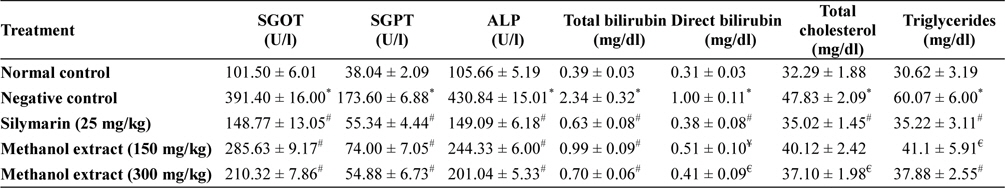
Treatment with toxic dose of acetaminophen resulted significant increase in serum SGOT, SGPT, ALP, total and direct bilirubin, total cholesterol and triglyceride level in animals of disease control group, which indicated that paracetamol in toxic dose impairs hepatic function. Higher dose of methanol extract produced significant beneficial effect. Level of above serum biochemical parameter found near to normal in extract (300 mg/kg) treated group (Table 1).
[Table 1.] Hepatoprotective activity of methanol extracts of L. asiatica leaves
Hepatoprotective activity of methanol extracts of L. asiatica leaves
Serum level of SGPT and SGOT are increases significantly in drug, toxin and chemic induced liver damage condition and considered as the most commonly used relied biomarker of hepatotoxicity. SGOT, SGPT, ALP releases into the extracellular space due to hepatocyte damages, which ultimately enter into circulation and thereby increase serum levels these enzyme in negative control group (Olaleye et al., 2010; Singh et al., 2011). In extract treated group the level of these enzyme was found less compare to negative control group, and in higher dose extract was found to maintained the level of these enzymes near normal which indicated that extract may preserved the integrity of hepatocellular membrane from the acetaminophen induced damage.
Hepatocyte damage or obstruction of excretory ducts of the liver spoils the capacity of liver to excrete normal amounts of bilirubin. Elevated levels of bilirubin may indicate severe dysfunction of liver. Total bilirubin considered as combination of indirect (nonhepatic) and direct (hepatic) bilirubin (Goel et al., 2012; McConnachie et al., 2007; Olaleye et al., 2010). This investigation showed that level of total and direct bilirubin were increased by the acetaminophen administration, but in extract treated group the level of direct and total bilirubin in the serum found significantly (
>
In vivo antioxidant activity
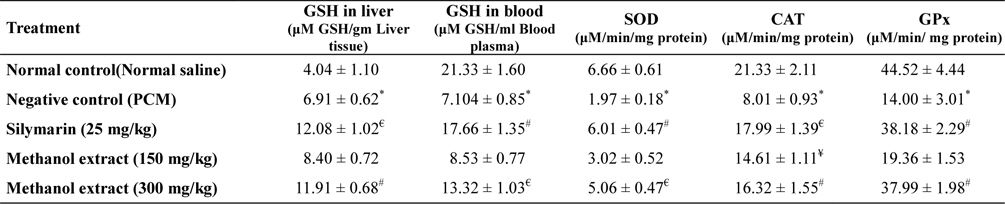
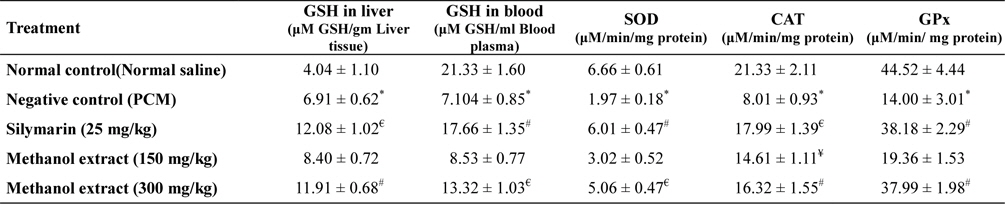
Administration of acetaminophen causes depletion of liver GSH and blood GSH content. SOD, CAT, GPx level also reduced significantly (
[Table 2.] In vivo antioxidant activity of methanol extract of L. asiatica leaves
In vivo antioxidant activity of methanol extract of L. asiatica leaves
SOD, CAT and GPx are the endogenous antioxidant enzymes that important to avert oxidative stress situation. SOD catalyzes the dismutation of superoxide to H2O2 and O2, while CAT converts H2O2 to water and molecular oxygen. GPx present in the cell cytoplasm eliminates H2O2 by coupling its reduction to H2O with oxidation of GSH (Sen and Chakraborty, 2011). Oxidative stress considered as one of the important factor of liver toxicity. NAPQI can increase the generation of different free radicals, reduces antioxidant enzyme level and causes lipid peroxidation which leads to oxidative stress (McConnachie et al., 2007; Olaleye et al., 2010). Extract treatment significantly increases level of endogenous antioxidant enzyme levels suggesting that extract possess significant
Metabolism of acetaminophen primarily occur in liver, where a small portion of acetaminophen (5 - 10%) in therapeutic dose metabolized via the hepatic cytochrome P450 enzyme to produce
Present work justifies the folk medicinal uses