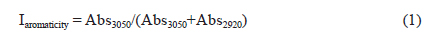Coal tar pitch (CTP) is a byproduct of the coking process and isotropic CTP is cheaper than other carbon fiber precursors such as polyacrylonitrile (PAN). Since CTP consists of a variety of different phenols and polycyclic aromatic hydrocarbons [1,2], it must be properly treated to obtain spinnable pitches. In order to achieve lower price and high performance pitch carbon fiber, CTP has been restructured by purifying processes [3,4], thermal and acid treatments [5], air blowing [6-9], thermal treatment [10], and modification with a polymer [11-13]. These treatments affect the properties of the carbon fiber precursor because they cause a considerable change in the chemical composition of pitches. As a result, the softening point (SP) and yield of the precursor pitch can be increased and the mechanical properties of the resultant carbon fiber can be improved.
To prepare an isotropic spinnable pitch, CTP should meet two important qualifications: it must have a high SP and should maintain isotropic characteristics or be transformed to a 100% meso-phase (anisotropic) pitch. To obtain excellent spinnability, CTP should have homogeneity in properties such as a short range of molecular weight distribution and viscosity. However, with treatment at high temperatures, isotropic CTP usually generates mesophase particles. To control the generation of a meso-phase, various methods have been used such as acid treatments to reduce reaction temperatures [5], and adding polymers to reduce or increase meso-phase formation [11-13]. Isotropic CTP is less costly than meso-phase CTP or PAN because of its low temperature manufacturing conditions. It is also suitable for spinning processes because of its low viscosity. However, since it has no directional characteristic, its carbon fiber has poor mechanical properties such as tensile strength and tensile modulus. The isotropic pitch fiber can be used in commonplace applications such as sporting goods and insulators [14].
Most previous studies on CTP have the limitation that modified CTPs were only analyzed but not applied to products such as carbon fiber. The goal of this study is to prepare a spinnable precursor pitch with proper properties for carbon fiber using isotropic CTP. To accomplish this goal, we investigated various treatments on CTP such as thermal treatment only and sulfuric acid treatment or nitric acid treatment before thermal treatment. The properties of the modified CTP and its fiber were analyzed by an elemental analysis, insoluble weight portion, thermogravimetric analysis (TGA), Fourier transform infrared spectroscopy (FT-IR), polarization microscope analysis, and scanning electron microscopy (SEM). Through these procedures, the optimal reaction conditions to prepare the spinnable precursor pitch at low production cost were demonstrated. In particular, this study focused on obtaining a high yield of precursor pitch with high SP and homogeneity for a continuous melt-spinning process.
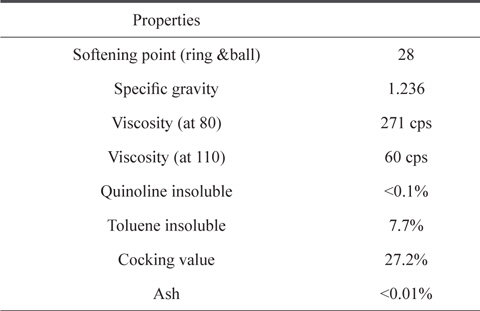
A commercial CTP provided by OCI Co. Ltd. (Korea) was used as the raw material. Its quinoline insoluble (QI) portion was eliminated by a centrifugal separator. The detailed information of CTP is presented in Table 1.
[Table 1.] Properties of raw coal tar pitch
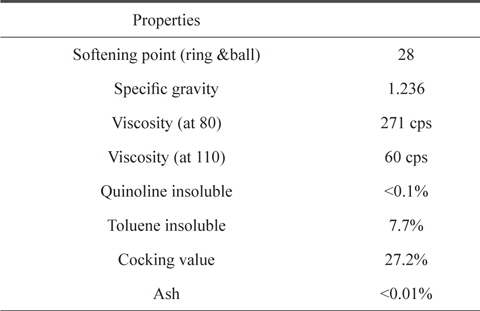
Properties of raw coal tar pitch
2.2.1. Thermal treatment
Thermal treated CTP is denoted as CTP-T. Thermal treatment of CTP was performed to compare the properties with those of acid treatments. 100 g of pitch was placed in a 500 mL round flask and heated from room temperature to 380°C-400°C at a heating rate of 5°C/min, and maintained with continuous stirring for 2 h. A vacuum pump with a cold trap was connected to the flask to eliminate emission gas.
2.2.2. Acid and thermal treatments
Sulfuric acid and nitric acid treated CTP are denoted as CTPS and CTP-N, respectively. The amounts of acid were determined such that the addition of 11 g of 95% sulfuric acid or 14 g of 60% nitric acid to 100 g of CTP was suitable to obtain a proper SP of the precursor pitch. Each acid reagent was injected to the melted CTP at 70°C. After mixing CTP with acid by stirring for 30 min, the mixture was heated to 280°C-300°C at a heating rate of 5°C/min and maintained with continuous stirring and applied vacuum for 2 h.
2.2.3. Nitric acid treatment with ethanol and thermal treatment
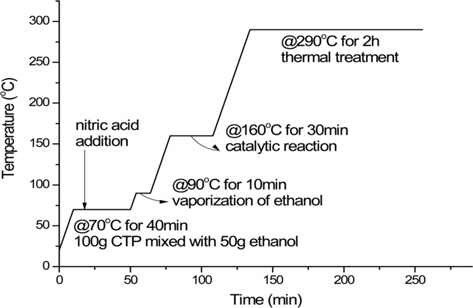
In order to improve the homogeneity, the CTP was mixed with ethanol before the consecutive nitric acid and thermal treatments. Nitric acid treated CTP with ethanol is denoted as CTP-NE. Since ethanol is an amphiphilic solvent, it may help obtain a more homogenous mixture of CTP and nitric acid. Fifty mililiters of ethanol was premixed with 100 g of pitch at 70°C. Then 14 g of 60% nitric acid was injected to the mixture and the following steps were the same as in the previous acid treatments.
2.3. Preparation of carbon fibers
Two precursor pitches (CTP-NE: SP236 and CTP-AB: SP284) were used for fiber spinning. The CTP-AB (CTP modified with air blowing and thermal treatments, SP 284°C, TI 65.8%, QI 21.4%) was provided from OCI Co. Ltd. and was also spun to compare the spinning and fiber properties with those of CTP-NE. The modification procedure of CTPNE: SP236 (nitric treated pitch with ethanol, SP 236°C) is represented in Fig. 1. Modified CTPs were spun into green fibers through a single-hole spinneret (diameter = 0.3 mm, L/D = 2) by pressurized nitrogen gas at a spinning temperature of 30°C-40°C abojavascript:;ve the SP and a winding speed of 500 m/min. The stabilization and carbonization processes were conducted using an electric tube furnace. Stabilization temperature was 10°C higher than the SP of the precursor pitch for 10 h under an air flow. In the carbonization process, the stabilized fibers were heated to 1000°C at a heating rate of 2.5°C/min and maintained for 3 min in a nitrogen atmosphere.
2.4. Characterization of precursor pitches and carbon fibers
Contents of carbon, hydrogen, and nitrogen for various pitch samples were measured by an elemental analyzer (EA1112; Thermo Fisher Scientific Co.). The furnace temperature was 900°C and the oven temperature was 500°C. Helium and oxygen flow rates were 140 mL/min and 250 mL/min, respectively. SP of the modified CTPs was measured by the Mettler method (KSM1808) using a dropping point system (DP70, Mettler Toledo Co.). The diameter of the nozzle is 6.35 mm. The starting temperature was about 20°C lower than the SP of the sample and the heating rate was 2°C/min. Two measurements were conducted for each sample and their average was taken.
A soxhlet extractor was used to measure insoluble mass portions of the modified CTPs. One gram of dried pitch sample was pulverized (~100 mesh) and located in a dried cellulose thimble filter (diameter 28 mm, height 100 mm). The pitch samples were extracted for 2 h by hexane and toluene. After extraction, the thimbles with remaining insolubles were situated in a dry oven and the solvents were evaporated for 24 h at 70°C before the weight measurement. QI could not be measured by the Soxhlet extractor because of quinoline’s high boiling point. The pitch sample was dissolved in warm quilonine several times and the solution with QI was filtered using an aspirator. It was then washed by acetone and the weight of residue was measured.
FT-IR spectra of the modified CTPs were obtained using a Fourier transform infrared spectrophotometer (DRS-800, Shimazu Co.). The spectra were obtained in a range of 400-4000 cm-1. A thermogravimetric analyzer (SDT 851, Mettler Toledo Co.) was used to demonstrate the carbon yield of CTP to carbon fiber and the approximate distribution of molecular weight. The modified CTPs were heated from room temperature to 1000°C at a heating rate of 10°C/min in a nitrogen atmosphere.
The viscosity of modified CTPs depending on temperature was measured using a stress controlled rheometer (MCR 300, Anton Paar Co.). The shear rate was 10 s-1 and the viscosity was measured with increasing temperature from the SP of samples in a nitrogen atmosphere. A SEM (S-3500N Hitachi Co.) was employed to analyze the diameter of carbon fibers with platinum coating. Optical microscopy images of the modified CTPs that were mounted with epoxy resin and polished were observed on a polarized light microscope (DM 2700P, Leica Co.)
3.1. Characterization of raw and modified pitches
3.1.1. Yield, SP, and elemental analysis
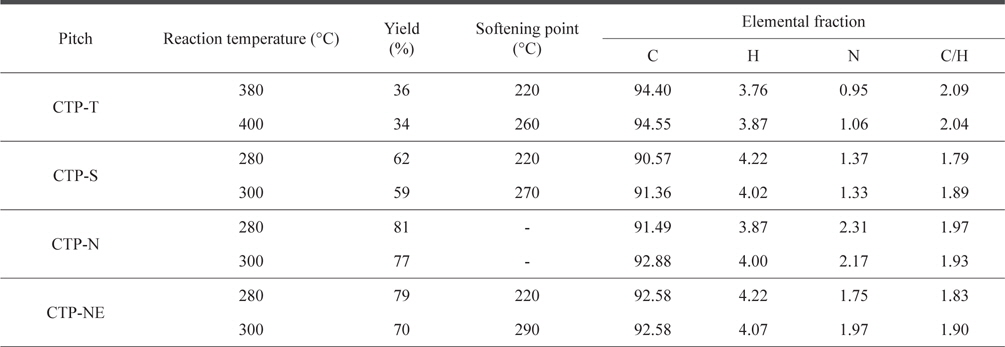
The yield, SP, and elemental content of various CTPs depending on the reaction temperature are shown in Table 2. When the raw CTP was only thermally treated, a relatively high temperature of between 380°C and 400°C was required to prepare a precursor pitch with a proper SP of 220°C-260°C. As higher temperature was applied, a larger amount of gas was produced and eliminated by the vacuum pump, resulting in an increased SP and decreased yield. The precursor pitch should have a high SP at least 180°C because the combination reaction between oxygen and pitch starts above 180°C on stabilization process. With increasing SP, higher stabilization temperature and shorter stabilization time can be used. As a result, the production cost of carbon fiber can be reduced because the stabilization step is the most expensive process. However, high SP over 300°C is not desirable due to difficulty of spinning. We therefore set a target SP range of 230°C-290°C. In the case of acid treatments, the precursor pitch with a proper SP range could be obtained at temperatures of 280°C-300°C, which were about 100°C lower than those for the thermal treatment only. Furthermore, the yield and SP of the precursor pitch increased dramatically with the acid treatments. The increased yield and low temperature of thermal treatment are very useful to lower the production cost of carbon products manufactured from the pitch precursors. Nitric acid treatment (CTP-N) showed even 15%-20% higher yields than sulfuric acid treatment (CTP-S). However, the SP of CTPN samples could not be measured by the Metter method because chemiof the low fluidity near the SP, indicating that the CTP-N was not spinnable. As the CTP was mixed with ethanol before the consecutive nitric acid and thermal treatments, the SPs of CTP-NE could be measured and were in the same range as those of CTP-S.
[Table 2.] Reaction temperature, yield, and softening point of thermal and acid treated pitches
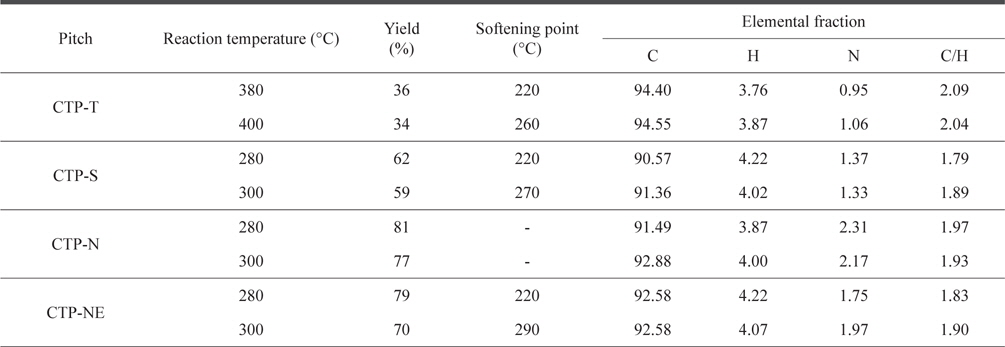
Reaction temperature, yield, and softening point of thermal and acid treated pitches
The elemental analysis showed that the CTP-T has a relatively high C/H ratio over 2.0 as compared with sulfuric and nitric acid treated CTPs. It appears that acid treatment increased hydrogen bonds. There was a small increase of nitrogen content in CTPN, indicating the formation of nitrogen functional groups during the polymerization of CTP with nitric acid [5].
3.1.2. Solubility results
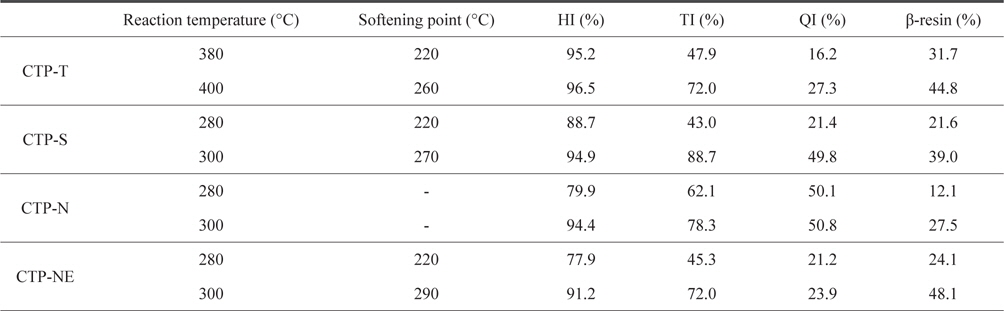
Insoluble mass portions of the modified CTPs are shown in Table 3. Beta resin refers to the portion of toluene insoluble (TI) minus QI. TI, QI, and beta-resin portions influence the formation of meso-phase. The existence of primary QI in isotropic pitch has an adverse effect on homogeneity with the creation of meso-phase particles and causes a viscosity differential. Isotropic QIs are asphaltene-like molecules scattered in modified CTP prepared by air blowing and acid treatment. Although the isotropic QI increases coke yield compared to untreated pitch, a high QI over 40% represents low fluidity becoming cokes [15-17]. Hexane insoluble (HI) of the thermal treated pitch (CTP-T) was over 95%. This means that the low molecular-weight potion of raw pitch was effectively eliminated by the vacuum or grew to high-molecular weight of polymer by the poly-condensation reaction. TI, QI, and beta resin of CTP-T treated at 400°C were much higher than those treated at 380°C. As higher reaction temperature was applied, a larger amount of low molecular weight portion disappeared by the activated poly-condensation reaction.
[Table 3.] Insoluble mass portion of thermal and acid treated pitches
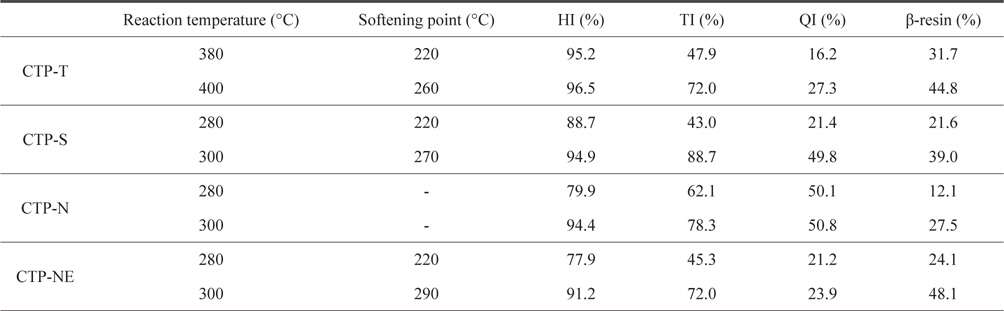
Insoluble mass portion of thermal and acid treated pitches
Insoluble portions of CTP-S were very dependent on the reaction temperature. This means that the sulfuric acid treatment is very difficult to control the optimum reaction temperature. TI and QI of CTP-S treated at 280°C were 43.0% and 21.4%, respectively, indicating that CTP-S had a large portion of low molecular compound. When the CTP-S was treated at 300°C, which was just 20°C higher, TI and QI increased more than twofold and beta resin increased from 21.6% to 39%. However, since QI was very high at about 50%, it was not suitable for spinning. The CTP-N also had a high QI of about 50% and beta-resin portions were relatively low. In addition to the previous SP analysis, these insoluble data meant that the CTP-N was not spinnable. To solve this problem, the CTP was mixed with ethanol before the consecutive nitric acid and thermal treatments. The CTP-NE showed slightly reduced TI and about 30% reduced QI as compared to the CTP-N. As a result, higher portions of beta-resin in a range of 24%-48% could be obtained and the obtained precursor pitch showed excellent spinnability, exhibiting continuous spinning for more than 30 min.
3.1.3. FT-IR results of modified pitches
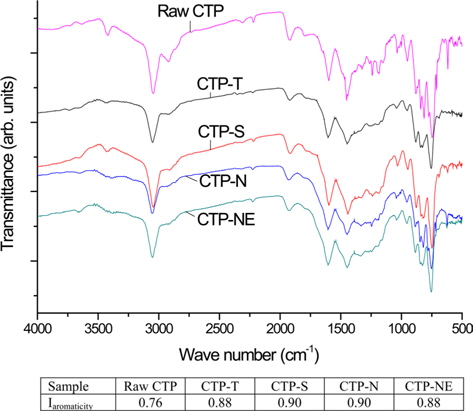
To examine the chemical structure of precursor pitch, their FT-IR spectra are shown in Fig. 2. Compared to the raw CTP, the modified CTPs made noticeable reductions of absorption at 2920 cm-1 but there are no significant differences among the various modified pitches. Because the formation of notable new bonds and the intensity change of existing spectra were not observed with the acid treatments, it was demonstrated that sulfuric and nitric acid helped the poly-condensation at low temperature, playing as the role of a catalyst, without remaining as chemical bonds. Some functional groups of the modified CTP were observed as aromatic (3050, 1161, 1505, 1052, 872, 811, 750, and 438 cm-1) and aliphatic (2955, 2920, 2855, and 1450 cm-1) groups. In particular, we can see the existence of an aromatic C-H stretch at 3050 cm-1 and the stretching of aromatic C=C at 1611-1470 cm-1 [18-20]. The aromaticity index of various CTPs were calculated by Eq. (1) reported by Guillén and co-workers [18].
High aromaticity is a well known characteristic of CTP as compared with petroleum pitch [21]. During the various treatments, the aromaticity index increased from 0.76 of raw CTP to 0.88-0.90.
3.1.4. Optical properties
Meso-phase formation was observed by polarized optical microscopy. The optical textures of differently treated CTPs are shown in Fig. 3. The thermally treated (CTP-T) developed many meso-phase particles due to the high reaction temperature of 380°C-400°C. The sizes of the meso-phase was less than 50 μm and the meso-phase particles did not grow forming a large size of lamellar. This texture makes the spinning process difficult. By applying both acid treatments before the thermal treatment, the meso-phase formation was suppressed due to 100°C lower reaction temperature. Thus, CTP-S, CTP-N, and CTP-NE were 100% isotropic precursor pitches. Their catalytic effect on the poly-condensation reaction enabled this low temperature reaction process, and thereby prevented the generation of mesophase [5].
3.1.5. Distribution of molecular weight and carbon yield
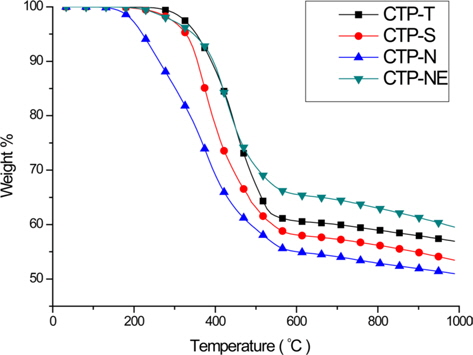
Although we tried nuclear magnetic resonance (NMR) obtain molecular weight distribution curve, the appropriate NMR solvent for CTP could not be found. The solubility of acetone was very low and peaks of deuterochloroform overlapped with the peaks of CTP. Thus, TGA was conducted to predict the distribution of molecular weight, as shown in Fig. 4. The low molecular weight portion of CTP was released in the form of a gas mixture at lower temperatures of 200°C-400°C. The gradual decrease in weight over a long range of temperature increase indicates that the distribution of molecular weight is broad. For the CTP-N sample, the weight decline started earlier than for the other samples, at around 200°C, and continued until 600°C. This broad distribution of molecular weight likely occurred due to the inhomogeneity of the nitric acid and CTP mixture during the poly-condensation reaction. In order to improve the homogeneity, the CTP was mixed with an amphiphilic ethanol solvent before the consecutive nitric acid and thermal treatments. With ethanol addition, the starting temperature of decline increased to 300°C and continued until about 550°C, indicating a narrower distribution of molecular weight. The narrower distribution of molecular weight means that the precursor has homogeneous properties. We can also infer the carbon yield form precursor pitch to carbon fiber by the TGA curves. When the modified CTPs were heated to 1000°C in a nitrogen flow, the CTP-N sample had the lowest carbon yield at 51%, and the carbon yields of the other CTPs were 53%, 57%, and 60% for CTP-S, CTP-T, and CTP-NE, respectively. Thus, a precursor pitch with a narrow distribution of molecular weight and a high carbon yield could be obtained by the ethanol and nitric acid treatment.
3.1.6. Viscosities of modified CTP
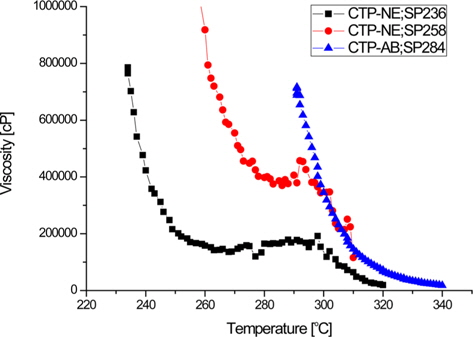
The viscosities of two types of spinnable pitch precursors are represented as a function of temperature in Fig. 5. CTP-AB and CTP-NE showed a similar viscosity of over 700 000 cP at their SP and the viscosities decreased sharply between SP and SP+15°C. In the case of CTP-AB, its viscosity declined to around 100 000 cP at SP+30°C. However, that of CTP-NE: SP236 did not change substantially from SP+20°C to SP+70°C and then decreased again, reaching 100 000 cP at SP+85°C. It was demonstrated that the catalytic effects of nitric acid accelerated the poly condensation and crosslinking, showing an irregular viscosity curve for CTP-NE.
The suitable temperature for pitch spinning is known as the point where the viscosity is about 80 000 cP at 3 bar. Thus, the spinning temperature of CTP-AB was chosen as 314°C (SP+30°C). However, since CTP-NE maintained a viscosity interval, this point was not suitable for spinning because of oxidation of the pitch and gas emission at high temperature. CTP-NE was spun at SP+35°C and higher pressure of more than 8 bar was required due to the high viscosity.
3.2. Properties of carbon fibers
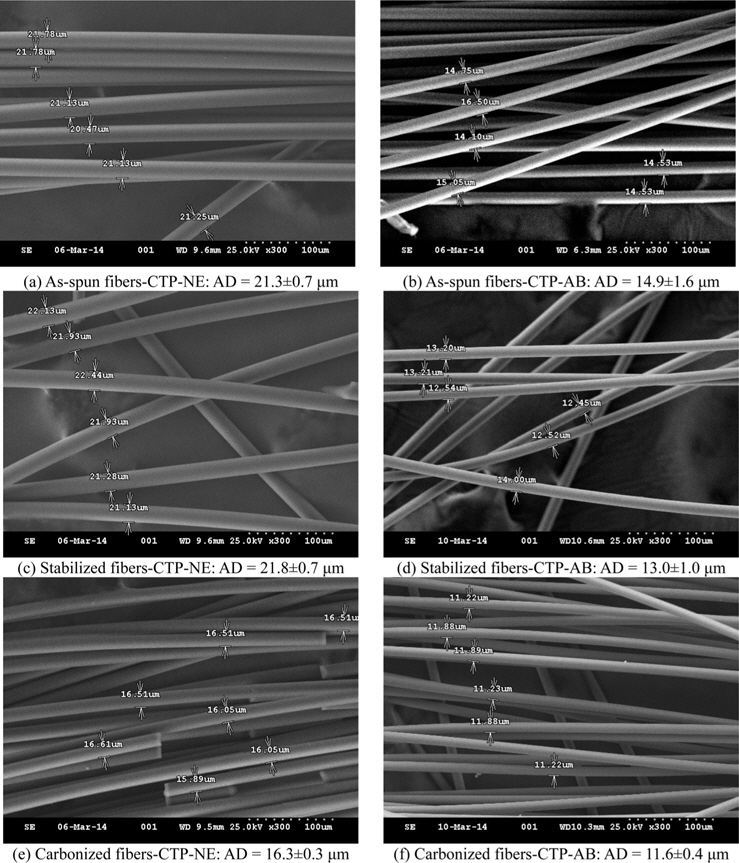
The SEM images of carbon fibers for CTP-AB and CTP-NE are shown Fig. 6. The average diameters (ADs) of as-spun fibers with CTP-NE and AB were 21.3 μm and 14.9 μm, respectively. This difference was caused by the differences in viscosity and spinning pressure. After stabilization, the AD of CTP-NE slightly increased to 21.8 μm because of oxygen permeation. On the other hand, that of CTP-AB decreased to 13.0 μm. Since CTP-AB underwent sufficient oxygen combination during air blowing treatment, the diameter decreased due to the gas emission during stabilization. During the carbonization process, their diameters decreased to 16.3 and 11.6 μm, respectively. The light gas of carbon fiber was eliminated by heating. The as-spun and stabilized fibers with CTP-AB appeared glossier in SEM images than those with CTP-NE and this difference was less pronounced after the carbonization.
In order to prepare a spinnable precursor pitch with proper properties for carbon fiber using isotropic CTP, we investigated various treatments on CTP such as thermal treatment only and sulfuric acid treatment or nitric acid treatment before thermal treatment. The thermal treated CTP required a relatively high reaction temperature of more than 380°C to prepare a precursor pitch with a high SP for carbon fiber and it had many mesophase particles that were created during application of high reaction temperature. When nitric acid or sulfuric acid treatment was conducted before the thermal treatment, a precursor pitch with a proper SP range could be obtained at reaction temperatures of 280°C-300°C. With the acid treatments, the yield and SP of the precursor pitch increased dramatically and the formation of a meso-phase was suppressed due to the lower reaction temperatures. However, in the case of the sulfuric acid treatment, it is too difficult to control optimal reaction conditions due to the sensitive temperature dependence. The nitric acid treated CTP also had problems of a broad distribution of molecular weight and low fluidity at spinning temperature due to the inhomogeneity of the nitric acid and CTP mixture during the poly-condensation reaction. By adopting the nitric acid treatment with ethanol, CTP achieved more homogeneous properties such as a short range of viscosity and a narrow distribution of molecular weight. The CTP with ethanol, nitric acid, and thermal treatments could be spun continuously, and their spinning and carbon fiber properties were compared to those of the air blowing and thermal treated CTP.