For the past forty years, the increase of food production has been based on the increased use of synthetic pesticides for pest control (Oerke, 2006). The adverse effect of synthetic pesticides on the environment and human health encouraged the investigation of biopesticides, known to be relatively less toxic than the synthetic pesticides (Saxena, 1989; Kim and Smith, 2001; Cerejeira
In an environment‐friendly agriculture, plant extracts have been perceived as a substitute material for synthetic pesticides, recognized as not alarming to human and animal health (Arnason
The instrumental methods for analyzing of cinnamaldehyde, cinnamylalcohol and salicylaldehyde using liquid chromatography (LC), gas chromatography (GC)‐mass spectrometry (MS) and LC‐MS in food and plants has been reported (Yajima
Contents of the active substances in thirteen commercial biopesticides containing cinnamon extract in Korea were not also studied.
Recently, the limonoids analysis method for the quality control of commercial biopesticides was reported by Lee
This study was aimed to define the marker compound of cinnamon based on the crop protection effect and to develop the clean‐up method and GC conditions for the determination of cinnamaldehyde, cinnamylalcohol and salicylaldehyde in biopesticides containing cinnamon extract. The study also conducted to investigate the contents of cinnamaldehyde, cinnamylalcohol and salicylaldehyde in commercial biopesticides.
Cinnamaldehyde (99% purity), cinnamylalcohol (97% purity) and salicylaldehyde (98% purity) were purchased from Sigma‐Aldrich, Saint Louis, USA. The high pressure liquid chromatography (HPLC) grade acetone was obtained from Tedia, Ohio, USA. The ENVI‐Carb solid phase extraction (SPE) cartridge (500 mg, 6 mL), HLB SPE cartridge (60 mg, 3 mL) and Florisil SPE cartridge (1000 mg, 6 mL) were purchased from Supelco, Philadelphia, Waters, Milford, and Applied Separations, Pennsylvania, USA, respectively. Thirteen (11, liquid type and 2, solid type) commercial biopesticides containing cinnamon extract were purchased from eight local companies in Korea.
>
Selection of marker compounds in biopesticides
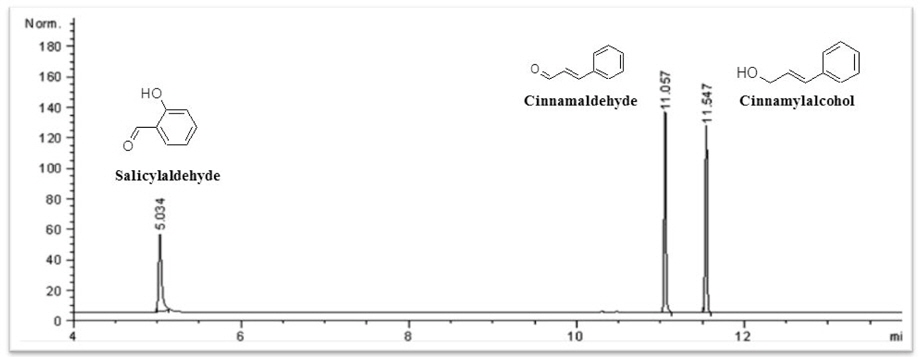
Three constituents in the cinnamon extract were selected as marker compounds in the biopesticides: cinnamaldehyde, cinnamylalcohol and salicylaldehyde. GC with flame ionization detector (FID) was used for content analysis.
>
Method for marker compounds analysis
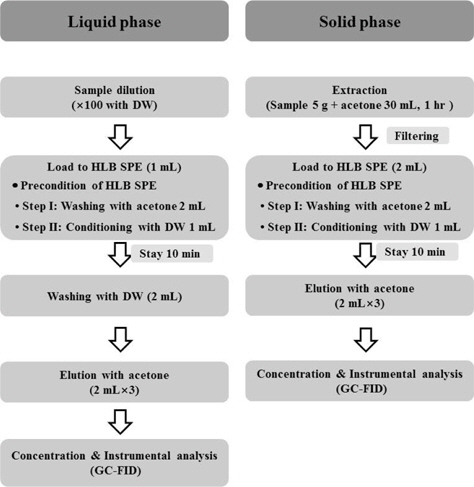
The marker compounds in biopesticides containing cinnamon extract were analyzed using the ENVI-Carb and HLB SPE cartridges. One mL of diluted biopesticide (100 times) with distilled water (DW) was placed in the ENVI-Carb SPE cartridge and eluted with 12 mL of hexane (3 mL×4). The organic phase was concentrated using an evaporator (Rotavapor R-124) purchased from Büchi, Flawil, Switzerland. Dried residue was then re-dissolved in 2 mL same solvent for GC analysis. The clean up using a HLB SPE cartridge was performed with the modified method of Lee
GC conditions for analyzing cinnamaldehyde, cinnamylalcohol and salicylaldehyde, were: system, Agilent 6890 series with FID (Agilent, Santa Clara, USA); column, RTX‐5 (30 m × 0.25 mm × 0.25 mm, RESTEK, Pennsylvania, USA); carrier gas, He (99.999%, 6 mL min‐1); injector temperature, 230℃; oven temperature, initial 70℃ (2 min hold), 3℃ min‐1, 93℃, 30℃ min‐1, 130℃, 3.5℃ min‐1, 140℃, 40℃ min‐1, 300℃; detector temperature, 300℃; and injection volume, 1 μl.
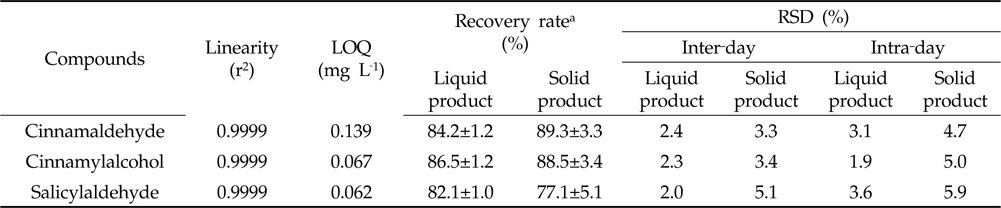
Sample preparation and analytical methods were validated in terms of linearity, limits of quantitation (LOQ), confirmatory and precision. The linearity of the calibration curves was evaluated by standard solution at concentrations 0.05, 0.1, 0.5, 1, 5 and 10 mg L-1, respectively, with five replications. The LOQ for cinnamaldehyde, cinnamylalcohol and salicylaldehyde were the concentration that produced a signal-to-noise ratio of 10. Confirmatory test was done based on the recovery assay results of samples spiked with all analytes at 6 mg L-1, with three replications. Recoveries were calculated by comparing the extracted amounts to the spiked with calibration curves of the cinnamaldehyde, cinnamylalcohol and salicylaldehyde, respectively. The intermediate precision, expressed as relative standard deviation (RSD, %), was determined through the replicates data (five replications) for three days at different levels.
Three substances, namely, cinnamaldehyde, cinnamylalcohol and salicylaldehyde were selected as marker compounds of cinnamon for their characteristics of crop protection. Commercial biopesticides generally contains a surfactant as additive. FID has also been used for the component analysis in matrix containing surfactant (Tacx and German, 1989; Fendlnger
>
Method of biopesticides analysis
In cleaning the biopesticides containing cinnamon extract, ENVI‐Carb and HLB SPE cartridges were used. Usefulness of these cartridges for analyzing the marker compounds in biopesticides was reported by Lee
The HLB SPE cartridge was used for the clean‐up of marker compounds such as azadirachtin A, azadiracchtin B, deacetylsalannin and salannin in commercial biopesticides containing neem extract (Lee
[Table 1.] Validation parameters
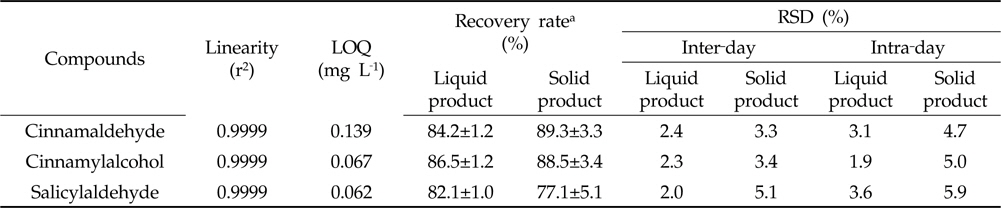
Validation parameters
The calibration curves of three substances were prepared form a concentration of 0.05‐10 mg L‐1 by its peak area, respectively. The coefficient of correlation of all calibration curves was 0.9999. The LOQs of cinnamaldehyde, cinnamylalcohol and salicylaldehyde were 0.062, 0.139 and 0.067 mg L‐1, respectively (Table 1). Confirmation of the analytical method was carried out in terms of recovery rates of the spiked sample having no cinnamon extract. Recovery rates of cinnamaldehyde, cinnamylalcohol and salicylaldehyde in liquid and solid‐type biopesticides obtained 82.1‐86.5 and 77.1‐89.3% by the established method, respectively (Table 1). The inter‐ and intra‐day precision methods were measured by recovery rates of cinnamaldehyde, cinnamylalcohol and salicylaldehyde for three days. The method was found effective since RSD percentages ranged from 1.9‐5.9% and below 15, the normal percent value (Table 1). These results showed that the analytical method including clean‐up procedure was suitable for determination of marker compounds contents in biopesticides containing cinnamon extract.
>
Marker levels in commercial biopesticides
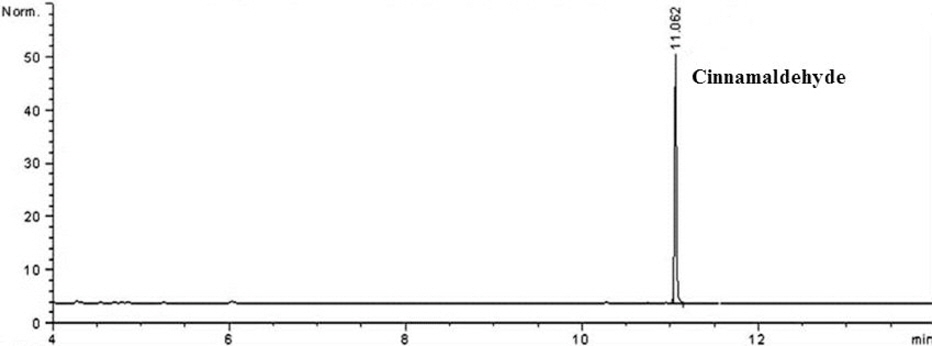
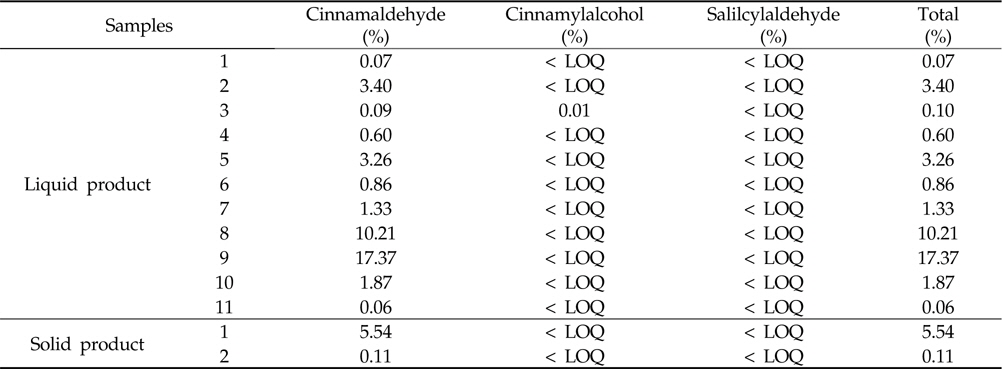
Marker levels of commercial biopesticides containing cinnamon extract were determined with the developed method. The developed method for cinnamaldehyde, cinnamylalcohol and salicylaldehyde analysis was also applied to commercial biopesticides containing cinnamon extract. Figure 3 presents the representative chromatogram of the cinnamaldehyde, cinnamylalcohol and salicylaldehyde contents in biopesticide samples.
Cinnamaldehyde, cinnamylalcohol and salicylaldehyde in all samples were detected at 0.06‐17.37%,
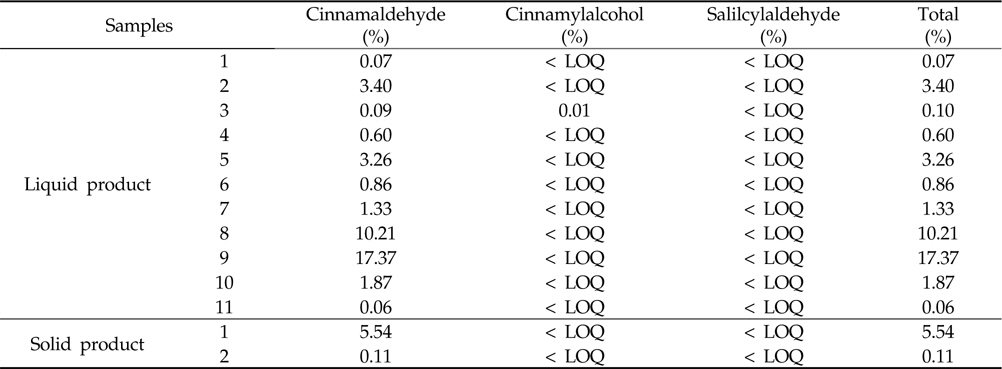
Cinnamaldehyde, cinnamylalcohol and salicylaldehyde contents of cinnamon extract using developed method in commercial biopesticides
The concentration of bioactive substances in commercial biopesticides, which were generally scattered for crop protection after 100‐1000 times dilution with water, was lower at more than 100 times. Therefore, the bioactive substance contents in the studied commercial biopesticide samples were expected to be lower than 1000 mg L‐1 for antimicrobial activity.
Cinnamaldehyde, cinnamylalcohol and salicylaldehyde, the characteristic constituents of cinnamon for crop protection, were selected as marker compounds. HLB SPE cartridge clean‐up method for the determination of three marker compounds in biopesticides containing cinnamon extract was developed and validated by GC-FID.
Results showed that cinnamaldehyde, cinnamylalcohol and salicylaldehyde showed good sensitivities in GC-FID and retention time was 11.06, 11.55 and 5.03 min, respectively. Recovery rates of the three marker compounds in liquid and solid‐type biopesticides were lower than 50% with the clean‐up method using an ENVI‐Carb SPE cartridge. Using the HLB SPE cartridge method, the recovery rates of marker compounds in liquid and solid‐type biopesticides were 82.1‐86.5 and 77.1‐89.3%, respectively.
The inter‐ and intra‐day precision was measured by comparing the recovery rates of cinnamaldehyde, cinnamylalcohol and salicylaldehyde for three days. And RSD ranged from 1.9 to 5.9% to be acceptable value for analytical criteria. These results showed that the experimental method including clean‐up and instrumental analysis were suitable for analyzing the cinnamaldehyde, cinnamylalcohol and salicylaldehyde contents in biopesticides containing cinnamon extract.
Cinnamaldehyde, cinnamylalcohol and salicylaldehyde contents in commercial biopesticide samples were detected at 0.06‐17.37%,
The initial findings on the analysis of cinnamaldehyde, cinnamylalcohol and salicylaldehyde in biopesticides have been proven to contribute in the manufacture and quality control of biopesticides for crop protection.