Frozen surimi is thawed, chopped, and mixed with water to control the protein concentration manufacturing of surimi-based products such as kamaboko. The mixture is ground with salt to solubilize myofibrillar proteins (Sano et al., 1988). This minced paste is heated to facilitate transformation of the viscous sol into an elastic gel (Numakura et al., 1990). Therefore surimi is the wet concentrate of myofibrillar protein prepared from fish mince in which most of the undesirable substances including sarcoplasmic protein, blood, fat, pigments and odorous materials have been removed by leaching with potable water. The surimi protein must be stabilized during frozen storage to produce high quality, good texture kamaboko. The fish muscle myofibrillar proteins, particularly myosin or actomyosin, which possess enhanced gel-forming, water holding and other functional properties, are very important for determining surimi gel strength. The surimi gelation related to the formation of cross-links between the myosin heavy chain induced by endogenous transglutaninase (Seki et al., 1990; Kumazawa et al., 1995) and thermal formation of non-covalent and disulfide bonds (Hossain et al., 1998). Therefore surimi gel strength is directly related to the degree of myofibrillar protein denaturation in fish muscle (Benjakul et al., 1997; 2003a; 2003b).
Surimi may lose its functional properties during frozen storage due to unfolding of the myofibrillar protein (mainly myosin), which exposes nonpolar amino acids and that become available for hydrophobic bond formation with same groups nearby. This process leads to protein aggregation (Shenouda, 1980), loss of gelling and water holding capacity (Carvajal et al., 1999) and decrease myosin ATPase activity (Suzuki, 1981). Cryoprotectants such as sucrose or sorbitol are required to prevent these undesirable changes in the myofibrillar protein and to preserve maximum functionality of surimi. It is well documented that these sugars work by stabilizing actomyosin, increasing surface tension (Arakawa and Timasheff, 1982) and the amount of bound water, preventing protein water loss (Buttkus, 1970), and maintaining protein solubility (Lim and Reid, 1991; Herrera and Sampedro, 2002). A mixture of sucrose and sorbitol is the preferred as cryoprotectant for fish surimi as both have excellent cryoprotective effects on fish myofibrillar protein (Lee, 1984; Yoon and Lee, 1990).
Phosphates are widely added to surimi with cryoprotectants such as sugar or sorbitol (Sultanbawa and Li-Chan, 2001) because phosphates improve the functional properties of the products by increasing water holding capacity of fresh fish and decreasing thaw loss of frozen fish (Chang and Regenstein, 1997). Adding phosphate to surimi increases pH, hence, it not only improves water holding capacity of the gel but also increases solubility of myofibrillar (Park, 2000). In contrast, phosphate slows down and inhibits setting of the surimi gel, as it chelates the Ca2+ ions (Julavittayanukul et al., 2006). Studies on the effects of phosphate on surimi have been mostly conducted with white muscle fish surimi, and information on dark fleshed fish is scarce.
Sand lance is a dark muscled fish species and is the main species caught off the Gangwon coast of Korea. This area has yielded >9,000 ton/year since 1993. Due to a lack of product innovation for sand lance, this fish mostly prepared as a plain dried product called gwuamegi. The best method for preparing surimi from this fish should be established to enhance the value of this fish and optimize it as fishery resource.
The aim of this study was to evaluate the effects of various cryoprotectant mixtures on the quality of sand lance surimi (SLS) during frozen storage. We investigated freeze-induced denaturation of SLS by determining Ca2+-ATPase activity and assessed the influence of denaturation on the textural properties of surimi by measuring the textural profile.
>
Surimi and surimi gel preparation
The SLS was prepared according to the method of Park et al. (1985) with a slight modification. Sand lance mince was suspended in cold (5°C) 0.5% NaHCO3 solution at a 1:5 ratio (w/v). This mixture was stirred gently for 15 min, and then the upper solution was removed. This procedure was repeated 292three times. The washed mince was dewatered by centrifugation at 15,000 rpm for 10 min. A 100-g aliquot of dewatered mince was mixed with designated various cryoprotectant mixtures (sorbitol, sucrose, sodium triphosphate, or their blends) in various concentrations (3, 5 or 8%) and ground with a grinder for 10 min at <10°C. These surimi mixtures were stored in PVC film at -30°C until further experiments.
Frozen surimi was thawed at 3°C for 15 min and cut into 1-cm thick slices to prepare the gel. The slices were ground with 2.5% NaCl for 10 min. A total of 150 g ground sample was encased in Krehalone casing film (Ø 3.0 cm) and incubated at 40°C for 30 min. Then the samples were heated at 90°C for 40 min and cooled down under tap water (15°C).
>
Determining Ca+2-ATPase activity
Freeze-denaturation of actomyosin in the SLS was carried out by measuring the Ca+2-ATPase activity index of myofibrils according to the method of Katoh et al. (1977). Myofibrils from frozen surimi were prepared according to the method of Katoh et al. (1977). About 4g of the partially thawed surimi was homogenized with three volumes of chilled 0.1 M KCl-20mM Tris-maleate buffer (pH 7.0) at 18,000 rpm for 30 s (three times) using a universal homogenizer (Tokyo Nihon Seiki Seisakusho Co., Tokyo, Japan). The homogenate sample was centrifuged at 1,400 g for 10 min at < 5°C. The sediment was washed with five volumes of same buffer, stirred, and centrifuged. This procedure was repeated until a clear supernatant was obtained, and the solution was use as the myofibrils. Protein concentration was determined by the Biuret method (Gornall et al., 1949) using bovine serum albumin as the standard.
Myofibrils (0.2-0.5 mg) were incubated at 25°C in final concentrations of 100 mM KCl, 25 mM Tris-maleate (pH 7.0), 5 mM CaCl2, and 1 mM ATP. This reaction was terminated after 5 min by adding chilled 30% TCA solution at a final concentration of 5%. Free inorganic phosphate was measured by colorimetry (Shimadzu UV-1800, Tokyo, Japan) in triplicate. The determinations of free inorganic phosphate content were made in triplicate. Ca+2-ATPase activity is expressed as μmoles inorganic phosphate released per mg protein per min.
The texture profile analysis (TPA) of the SLS gel was performed at ambient temperature with a rheometer (Fudoh, NRM-2010J, Tokyo, Japan) and a 1-kg cell according to the method described by Yoo (2011). The following textural parameters were calculated from the TPA curves according to the method described by Yoo (2011): hardness at 70% deformation, brittleness, elasticity and cohesiveness.
A surimi gel-folding test was conducted using the method described by Yoo (2011) to investigate the binding structure of the surimi gels. The folding tests were performed by slowly folding a 3-mm slice of surimi gel in half, and then in half again to examine structural rupture of the surimi gel slice. The number of folds required to crack the slice was scored from 1.00 - 5.00 and assigned to 5 classes: AA, A, B, C or D:, where AA (score 5.00) is the best quality and D (score 1.00) is poorest.
SLS gels were evaluated for texture, taste and overall acceptance by 15 untrained panelists. A 5-point hedonic scale, in which a score of 1 = not like very much, 3 = neither like nor dislike and 5 = like extremely, was used for the sensory evaluation.
Every experiment was replicated three times. Experiments were conducted on two type of cryoprotectants (sucrose, and sorbitol) with four concentrations (0%, 3%, 5%, and 8%) of sorbitol and three concentrations (0%, 3%, and 5%) of sucrose. Mixtures of 3% sucrose and 3% sorbitol (SOS) as well as 3% sorbitol with 0.2% sodium triphosphate compound (SOP) were also tested. The least significant difference at 5% was applied to define a significant difference between mean values. All analyses were performed using SAS software V8.1 (SAS Institute, Cary, NC, USA).
>
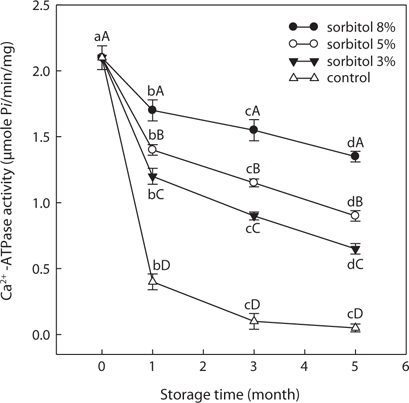
Effects of various cryoprotectants on the SLS Ca2+-ATPase activity
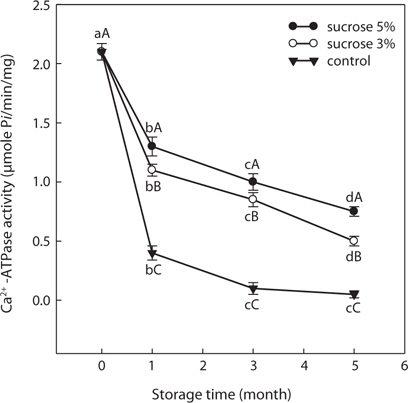
SLS Ca2+-ATPase activity of the control group (without cryoprotectant) was decreased significantly after first month in frozen storage and decreased gradually until only 2.38% of initial activity (2.10 μmol/min/mg) was detected after 5 months. Ca2+-ATPase activity of SLS with sorbitol (3, 5, and 8%) or sucrose (3% and 5%) as cryoprotectants declined significantly (
These results show that sorbitol or sucrose prevented the decrease in SLS Ca2+-ATPase activity and that these compounds had a cryoprotective effect on sand lance muscle protein during frozen storage.
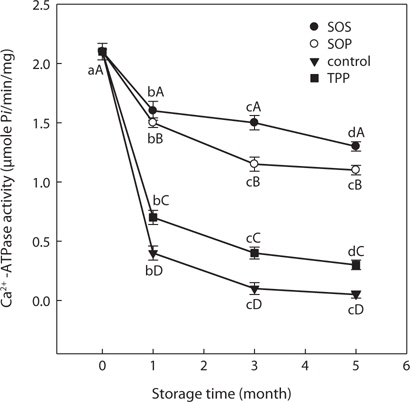
Sorbitol and sucrose are common cryoprotectants widely used in surimi industry. The mixture of both is preferable, as it has excellent cryoprotective effects on fish myofibrillar proteins (Lee, 1984; Yoon and Lee, 1990) and is a less sweet with fewer calories in the final product compared with sucrose only as a cryoprotectant. SLS Ca2+-ATPase activity with SOS and SOP was monitored to assess the effects of cryoprotectant combinations on freeze denaturation of SLS myofibrillar protein during storage at -30°C. Residual SLS Ca2+-ATPase activity with added SOS decreased gradually and was 61.90% of initial activity (from 2.1 to 1.3 μmol/min/mg) after 5 months of storage (Fig. 3). This value was higher than that of the 5% sorbitol or 5% sucrose only treatments (Figs. 1 and 2). Therefore, the mixture of sorbitol and sucrose had a stronger cryoprotective effect on SLS myofibrillar protein than did sorbitol or sucrose alone. Residual SLS Ca2+-ATPase activity with added SOP decreased to 1.10 μmol/min/mg after 5 months of storage. Residual SLS Ca2+-ATPase activity with added SOP exhibited a significantly higher value compared with 5% sorbitol alone.
Among all blends, SOS resulted in the highest (
>
Effects of cryoprotectants on the textural properties of the SLS gel
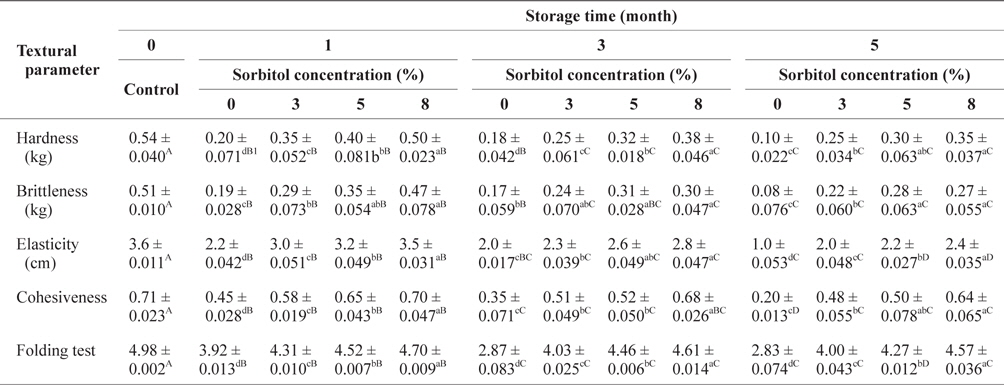
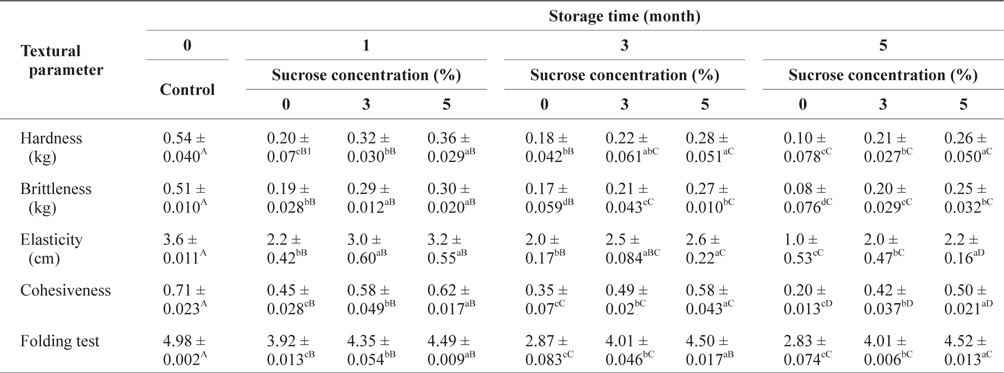
The changes in textural parameters of SLS gels prepared with various concentrations of sorbitol during storage at -30°C are shown in Table 1. The hardness, brittleness, and elasticity values as well as the folding test in all SLS gels increased significantly (
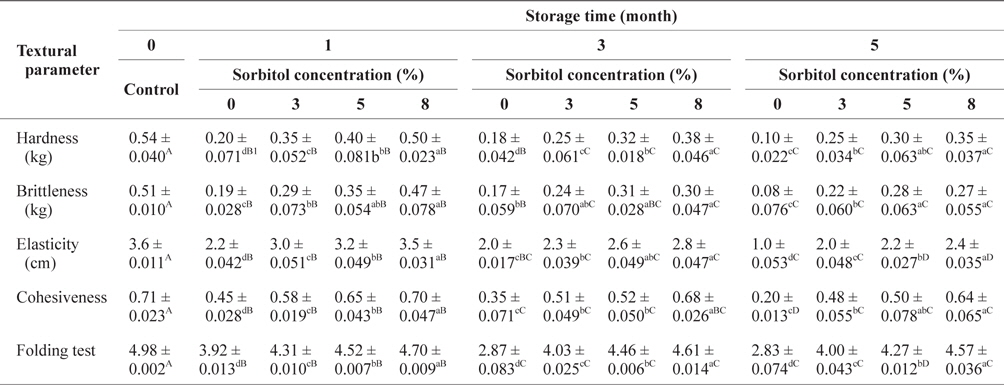
Changes of textural parameters of SLS gel prepared with various concentrations of sorbitol during storage at -30°C
Table 2 shows the changes in SLS gel textural parameters in the different sucrose concentrations treatments during storage at -30°C. The hardness, brittleness, elasticity and cohesiveness values as well as the folding test results of gels increased significantly (
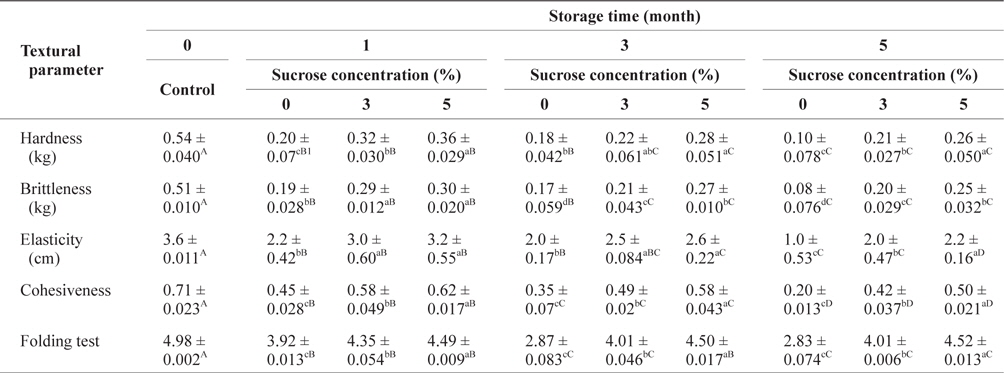
Changes of textural parameters of SLS gel prepared with various concentrations of sucrose during storage at -30°C
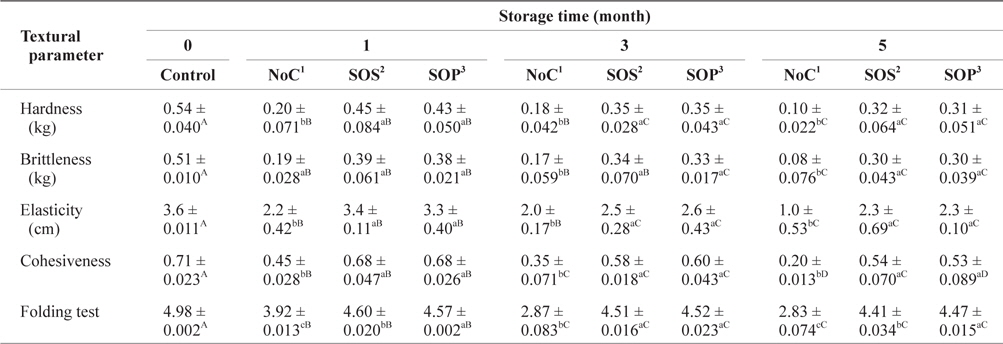
The changes in the textural parameters of SLS gels prepared with various cryoprotectant blends during storage at -30°C are listed in Table 3. The control hardness parameter of SLS surimi decreased significantly (
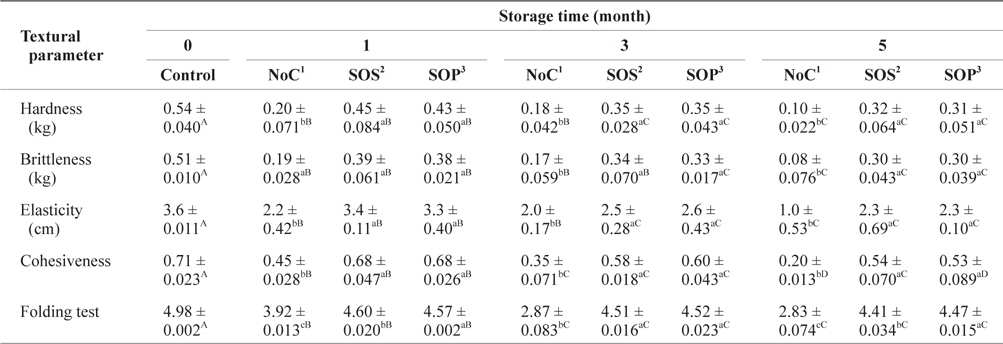
Changes of textural parameters of SLS gel prepared with various cryoprotectant blends during storage at -30°C
>
Effects of cryoprotectant on the SLS gel sensory evaluation
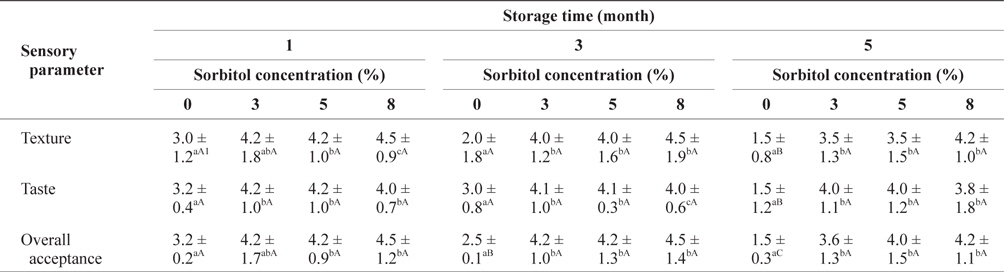
The preference scores for the SLS gels prepared with different quantities of sorbitol during storage at -30°C are shown in Table 4. The texture and overall acceptance scores of the SLS gels with 8% sorbitol at the same storage time tended to be the higher than those with the 3 or 5% concentrations, but the differences were not significant. The taste of SLS prepared with 8% sorbitol was less preferable compared with that of SLS with 3 and 5% sorbitol. The overall acceptance scores of the SLS gel with sorbitol did not decrease remarkably during storage. However, the scores for the SLS gels without sorbitol decreased significantly (
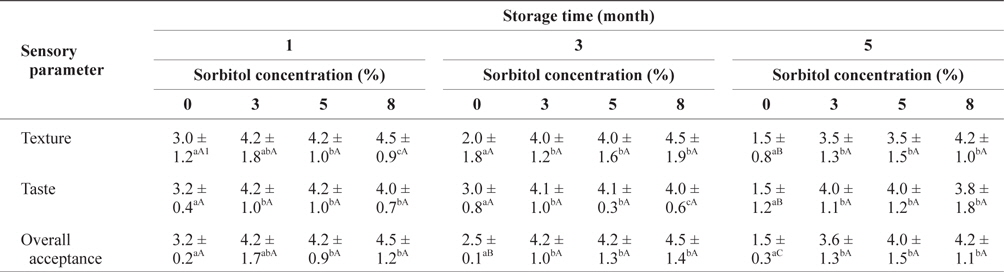
Sensory evaluation of SLS gel prepared with different concentrations of sorbitol during storage at -30°C
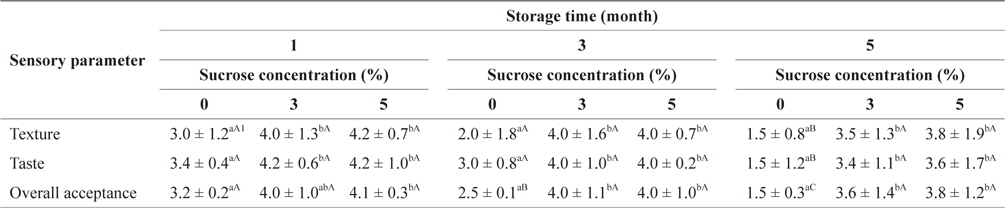
Table 5 shows the preference scores for the SLS gels prepared with different quantities of sucrose during storage at -30°C. After 1 or 3 months of storage, preference scores based on texture, taste, and overall acceptance of the SLS gels tended to increase in accordance with sucrose concentration, but the difference was not significantly different. Adding 3 and 5% sucrose resulted in significantly (
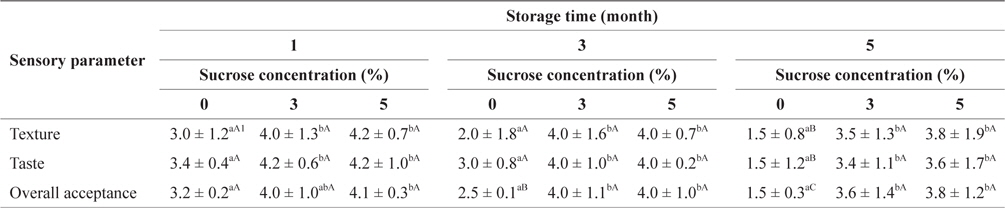
Sensory evaluation of SLS gel prepared with various concentrations of sucrose during storage at -30°C
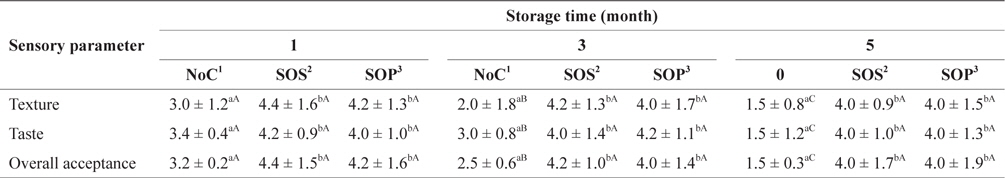
Table 6 shows the preference scores for SLS gels prepared with various cryoprotectant mixtures during storage at −30°C. All SLS gel preference scores for texture, taste and overall acceptance prepared without cryoprotectant (NoC) decreased significantly (
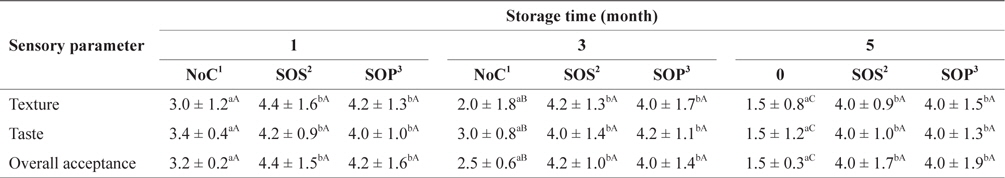
Sensory evaluation of SLS gel prepared with various cryoprotectants blends during storage at -30°C