In nature, parasitoids are known to employ a number of adaptive reproductive strategies that should lead to the production of more progeny with individuals possessing superior fitness qualities such as bigger body size, longer survival, efficient host searching ability, good adaptability to field environments, and so on. As a prelude to adaptive reproductive strategy, host age and size, parasitoid age and size, host availability, host-parasitoid ratio, presence of other parasitoids, environmental conditions, especially, temperature, assume paramount importance (Godfray, 1994). Before oviposition on or in a host, the parasitoid adjusts its clutch in such a way that it matches with quantity and quality of host resources. This type of oviposition behaviour is encountered by the parasitoid as long as host availability is ensured without any difficulty. However, under the conditions of reduced host availability, the parasitoid would allocate more eggs / larvae per host than can facilitate their successfull development on or in it. Such a condition is referred to as superparasitism, which is often considered as maladaptive as it can lead to the loss of offspring and search time. In contrast, Jacques et al.(1990) reported that superparasitism is an adaptive strategy for parasitoids in a number of situations as it creates a situation for them to oviposit even under limited availability of host despite the fact that such a behaviour leads generally to reduced fitness returns as a tradeoff mechanism between the number of offspring developed from a host and their size.
Nesolynx thymus Girault (Hymenoptera:Eulophidae) an ectopupal gregarious parasitoid of the tachinid (uzi) fly, Exorista bombycis (Louis) (Diptera:Tachinidae), which is an endolarval parasitoid of the mulberry silkworm, Bombyx mori L. Incidentally, E. bombycis inflicts a cocoon yield loss of 10 − 20% in silkworm rearings in the southern sericultural belt comprising Karnataka, Andhra Pradesh and Tamil Nadu in India. Owing to the possession of a number of desirable attributes of a biocontrol agent, N. thymus has been recommended for biological control of E. bombycis. The parasitoid when released for suppression of E. bombycis population in silkworm rearings, it often fails to perform to its potential presumably due to the presence of male biased population, reduced female longevity, poor host searching ability, and/or decreased ovposition. The occurrence of such parasitoid adults in a mass produced population could be due to superparasitism of the host pupae by the parasitoid. Therefore, an attempt has been made in the present investigation to record the impact of competition among the conspecific females for parasitization of host (conspecific superparasitism) on the developmental as well as morphological characteristics of N. thymus.
The adults of the experimental insect N. thymus were procured from the laboratory stock-cultures maintained in the Department of Studies in Sericulture Science, University of Mysore, Mysore. The host puparia, hereinafter called pupae, were obtained by allowing the post-parasitic maggots of E. bombycis collected from the silkworm cocoon markets in Karnataka (India) to pupate at room temperature (23 − 28℃). To study the influence of conspecific superparasitism on various developmental and morphometric parameters of N. thymus, 2 day-old N. thymus adults were provided with 3 dayold pupae of E. bombycis for parasitisation at host-parasitoid ratios of 1:1 to 1:10. The parasitoid adults were allowed to parasitize the host pupae for a period of 3 d when they were fed honey 50%. Thereafter, they were separated from the host pupae and the latter were kept for parasitoid emergence. Each treatment consisted of 10 replications.
Following the emergence of the parasitoid progeny (first generation) number of pupae parasitized, developmental duration, sex-wise and total adult recovery, sex ratio, adult size and adult female longevity were recorded / calculated. Subsequently, the gravid female (2 day-old) emerging at different treatments (1:1 to 1:10) were offered four 3 dayold uzi pupae for parasitization for 3 d. After the emergence of parasitoid progeny (second generation) observations were recorded on the following: number of pupae parasitised, developmental duration, sex-wise and total adult recovery, and sex ratio. The longevity of first generation N. thymus females was determined by maintaining 10 replications each with 10 adults. The adults were fed honey 50% and their mortality was recorded at 24 h intervals. The size of the parasitoid adults was measured by selecting randomly 10 adults of each sex using an ocular micrometer at 40x.
The results were analyzed statistically (SPSS Package) by one way ANOVA followed by DMRT for level of significance. Karl Pearson’s correlation co-efficients were also estimated to know the correlation between the ovipositing parasitoid density and reproductive as well as morphometric characteristics of N. thymus.
Developmental duration
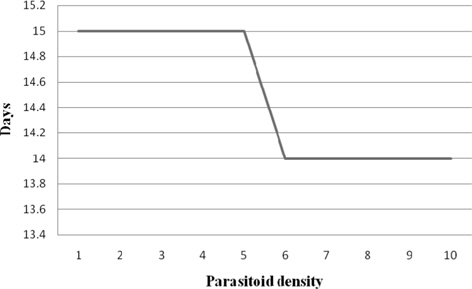
The parasitoid developmental duration recorded at treatments 1:1 − 1:5 and 1:6 - 1:10 were 15.00 and 14.00 d, respectively. The correlation between the parasitoid developmental duration and the parasitoid density was significant and negative (-0.867) (Fig. 1).
Progeny recovery per pupa
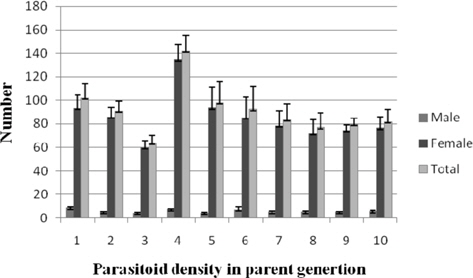
A progressive increase in male progeny of N. thymus was observed as the parasitoid density increased, with minimum and maximum values being 3.55±0.53 (1:1) and 68.85±1.14 (1:8), respectively. It increased significantly (p ≤ 0.01) from treatment 1:3 (27.66±3.59) onwards with comparable values up to 1:7 (39.14±5.12) beyond which there was a further increase. The correlation between the parameter and the parasitoid density was significant and positive (0.808). The parasitoid female progeny among the treatments ranged between 105.00±2.05 (1:8) and 75.87±4.95 (1:5). Nonsignificant correlation between parasitoid female progeny and parasitoid density was recorded. The parasitoid total progeny recovered per host pupa increased with increase in parasitoid density. The results varied from 173.85±2.89 (1:8) to 84.44±4.97 (1:1) among the treatments. Significantly greater recovery (p ≤ 0.01) was obtained at most treatments from 1:4 (114.87±12.21) to 1:10 (132.77±6.58). Significant positive correlation (r = 0.549) was also observed between the total progeny recovery per pupa and density of the ovipositing parasitoid (Fig. 2).
Progeny production per female
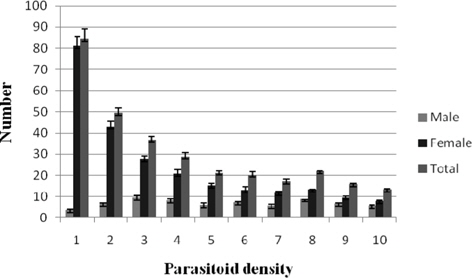
Male progeny per female fluctuated between 9.22±1.19 (1:3) and 3.55±0.53 (1:1) among the treatments. It was significantly higher (p ≤ 0.01) in majority of the treatments at 1:2 (6.50±0.54) − 1:10 (5.50±0.54). No definite trend of either increase or decrease in the parameter was recorded. Correlation between the parameter and the parasitoid density was realized when the data were nonsignificant. The production of parasitoid female progeny per female decreased with increase in the density of ovipositing females and the decrease was progressive and highly consistent. The results for the parameter ranged between 80.88±4.59 (1:1) and 7.77±0.36 (1:10). Significant decrease (p ≤ 0.01) in female progeny production was recorded from treatment 1:2 (42.92±2.51) onwards. When the data were subjected to correlation analysis, significant negative correlation (r = -0.782) was obtained between parasitoid female production per female and the parasitoid density. Maximum and minimum values for total progeny per female were noticed at 1:1 (84.44±4.97) and 1: 10 (13.27±0.65), respectively. The total progeny production per female diminished progressively and consistently with increase in the density of the ovipositing N. thymus. The values for the parameter decreased significantly (p ≤ 0.01) from the treatment 1: 2 (49.42±2.83) and upward. Further, the results at most treatments from 1:5 (21.05±1.81) to 1: 10 were found comparable. In addition, significant negative correlation (r = -0.790) was realized between the parasitoid density and the parasitoid total progeny production per female (Fig. 3).
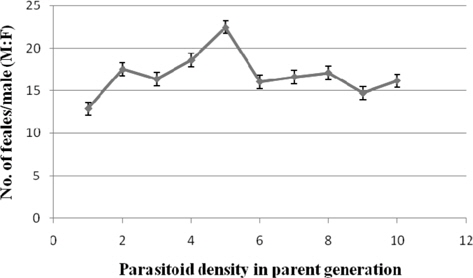
Sex ratio
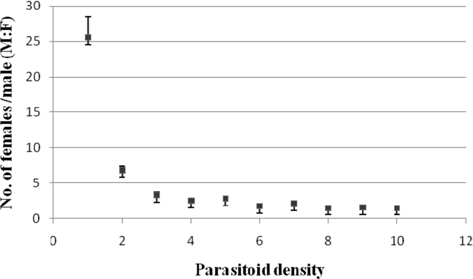
The parasitoid sex ratio was highest at 1:1 (1:25.49±2.96) and lowest at 1: 8 (1:1.52). It decreased sharply at 1:2 (1: 6.82±0.57) from where the decline was gradual and more consistent. The sex ratio was significantly higher (p ≤ 0.01) at 1:1 followed by 1:2 compared to the remaining treatments. Further, significant negative correlation (r = -0.615) was noticed between the parasitoid sex ratio and the density of ovipositing N. thymus (Fig. 4).
Female longevity
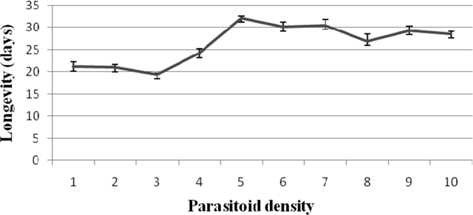
The survival duration was longest at 1: 5 (32.20±0.37 d) and shortest at 1: 3 (19.40±0.40 d). The correlation between the parameter and parasitoid female density was significant and negative (r = -0.540) (Fig. 5).
Body length
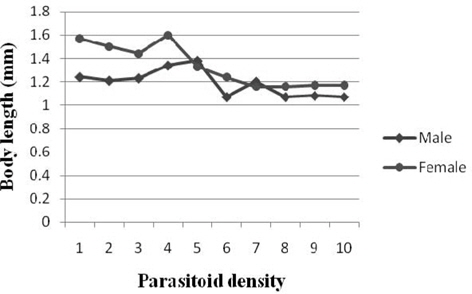
A decreasing trend in the body length of male was observed among the treatments with the results ranging between 1.38 mm (parasitoid density 5) and 1.07 mm (6, 8 and 10). Comparison of mean values indicated significantly higher measurement at 5 followed 4 (1.34 mm). Significant negative correlation (r = -0.592) was observed between parasitoid density and the male body length. The female body length decreased almost consistently with parasitoid density. The values for the parameter fluctuated from 1.60 mm (4) to 1.16 mm (7 and 8) among the treatments and they varied significantly among most treatments. Significant and negative correlation was observed between parasitoid density and body length of male (r = -0.592) and female (r = -0.850) (Fig. 6).
Head width
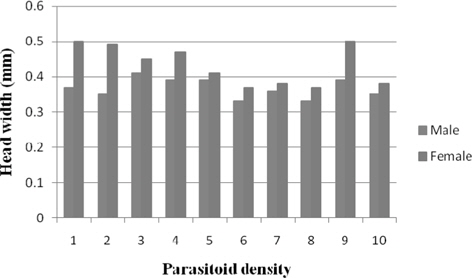
Head width of male N. thymus ranged between 0.41 mm (3) and 0.33 mm (8) among the treatments. Significantly greater (p ≤ 0.01) and comparable male head width was obtained at 3 − 5 (0.39 mm). A consistent decline in the female head width was noticed with increase in parasitoid density among the treatments. It decreased significantly (p ≤ 0.01) at parasitoid density 2 (0.49 mm) followed by 4 (0.47 mm), 3 (0.45 mm), 5 (0.41 mm), and 6 (0.37 mm. Besides, significant negative relationship was observed between parasitoid density and head width of male (r = -0.20) and female (r = -0.418) (Fig. 7).
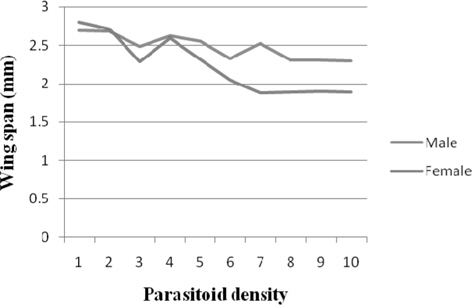
Wing span
The results for male wingspan fluctuated between 2.69 mm(1) and 2.30 mm (10). The male wingspan diminished more or less consistently with parasitoid density. Comparable and significantly higher (p ≤ 0.01) wingspan was realized at 1, 2 (2.68 mm), and 4 (2.62 mm). There was more or less consistent decrease in the female wingspan with parasitoid density. It was significantly higher (p ≤ 0.01) at 1 (2.80 mm). Further, significant negative correlation was recorded between parasitoid density and the wingspan of male (r = -0.802) and female (r = -0.896) (Fig. 8).
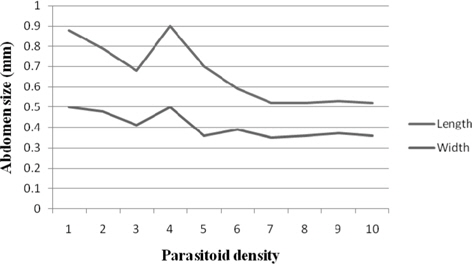
Female abdomen size
The results for N. thymus female abdomen length ranged from 0.90 mm (4) to 0.52 mm (7 − 10). Comparison of mean values among the treatments revealed comparable and significantly greater (p ≤ 0.01) length at 1 (0.88 mm) and 4 followed by 2, 5,3, 6, and 7. When the data for the parameter were subjected to correlation analysis, significant negative relationship (-0.831) was realized.
Statistically alike and significantly higher (p ≤ 0.01) values were scored for abdomen width at 1 and 3 (0.50 mm) and 2 (0.48 mm) as against the remaining treatments. No significant correlation was established between the female abdomen width and parasitoid density (Fig. 9).
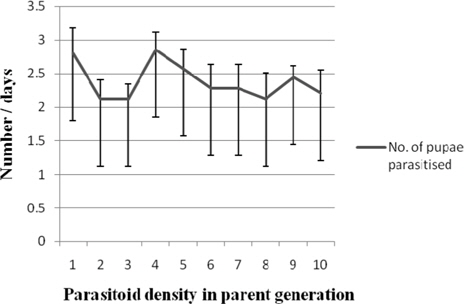
Number of pupae parasitiszed
Nonsignificant variation in the number of host pupae parasitised by first generation N. thymus was observed among the treatments with the values ranging between 2.85±0.26 (1:4) and 2.12 (1:2, 1:3, 1: 8) (Fig. 10).
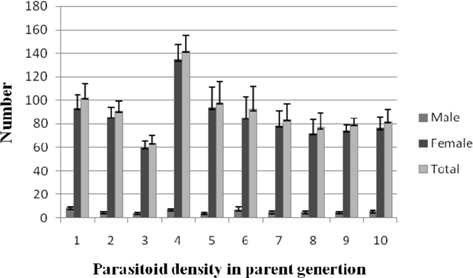
Progeny production
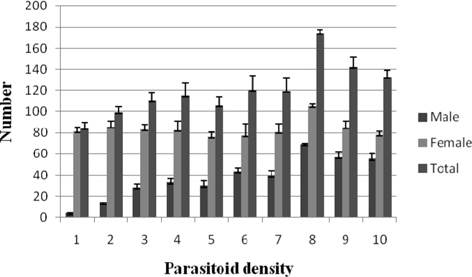
The male progeny per female among the treatments fluctuated from 8.40±1.39 (1:1) to 4.12±0.71 (1: 3). Statistical analysis of the data for the parameter did not revealed significant variation. Female progeny per female was significantly greater (p ≤ 0.01) at 1: 4 (134.57±12.58) as against the remaining treatments consisting of comparable values. The total N. thymus progeny at 1:4 (141.85±13.13) was significantly higher (p ≤ 0.05) among the treatments. The results fluctuated between 90.87±8.35 (1:2) and 63.87±6.44 (1:3) (Fig. 11).
Sex ratio
Among the treatments, it varied from 1:22.48±1.35 (1:5) to 1: 14.70±1.12 (1:9). The parasitoid sex ratio observed at 1: 5 while being comparable with that at 1:2 (17.51±1.25) and 1:4 (1:18.57±0.88) was significantly greater than the remaining treatments (Fig. 12).
Nonsignificant correlation was noticed between the parasitoid density and the reproductive performance of first generation adults when the data were subjected to statistical analysis.
The Hymenopteran parasitoids have the unique ability to regulate clutch size in relation to age, size, and quality (Godfray, 1994, Waage, 1986) and parasitisation status of host (parasitised or otherwise) (Ardeh et al., 2005). Sometime, the ability to regulate clutch size fails, especially when the host availability is scarce. As a result, the parasitoid is compelled to allocate more eggs to a host than that can permit its successful development, a condition commonly known as superparasitism. Possibilities of a single host being parasitized by more than one species of parasitoid are also not uncommon in nature, a situation commonly referred to as multiple parasitism. Though on many occasions over- allocation of progeny is avoided by the parasitoids, they are forced to allocate egg load to a host in excess of that which can facilitate successful development of the parasitoid individuals on the available host resources, thereby resembling superparasitism. A number of researchers attempted to study the effect of superparasitism on reproduction-related aspects of parasitoids. Singh and Sinha (1995) reported that number of hosts parasitized and number of Pediobius sp. adults emerging from parasitized hosts increased with number of the parasitoid females parasitizing the host. Further, the number of hosts parasitized and number of the parasitoid adults emerging per female decreased with the parasitoid density. Similarly, number of eggs laid per host was increased and number of eggs laid by each female decreased with parasitoid density in Psix striaticeps (Singh and Thangavelu, 1994). Biswas and Singh (1995) too have reported the increase in total progeny and decrease in mean number of progeny per mother with increase in parasitoid density in Lysiphlebus delhiensis. In Exorista japonica Townsend, the mean number of adults emerging from host increased up to a clutch size of 15 and thereafter it decreased (Nakamura, 1995). The author also reported that the body size of the emerging adults decreased sharply up to a clutch size of 6 and gradually thereafter, apart from recording non-significant variation in the longevity of the offspring. Increasing number of females per host patch has been reported to enhance the proportion of males in L. delhiensis (Biswas and Singh, 1995) and in a number of gregarious parasitoids (Suzuki and Iwasa, 1980) and to reduce percentage of female offspring in Campoletis chlorideae Uchida (Kumar et al., 2000).
In the present investigation, attempts to record the influence of parasitoid density on the reproduction of N. thymus on a single pupa of E. bombycis revealed significant positive correlation for male and total progeny and a significant negative correlation for production of female and total progeny per female and sex ratio with parasitoid density. The results with respect to number of adults produced and number of adults per female are in consonance with those of Singh and Sinha (1995), Singh and Thangavelu (1994), and Biswas and Singh (1995). The significant negative correlation between N. thymus developmental duration and its density can be understood as a reflection of significant positive relationship between parasitoid male progeny and parasitoid density. It is a well-known fact that the developmental duration of parasitoid males is generally shorter than females. Obviously, the development of more male progeny of N. thymus might have contributed to the reduction in the mean developmental duration with increase in parasitoid density. Insofar as the parasitoid adult size was concerned, all the morphological parameters, except female abdomen width, revealed significant negative correlation with parasitoid density. However, female abdomen width did not show any relationship with parasitoid density. The above results substantiate the observations of Nakamura (1995). From the results, it becomes quite apparent that increased progeny allocation by the parasitoid caused the reduction in body size of progeny, possibly as a consequence of competition for host resources among the developing progeny.
Despite being smaller when developed at higher parasitoid densities, the first generation parasitoid females exhibited reproductive efficiency generally comparable to those developed at lower parasitoid densities. It is, therefore, apparent from these observations that though the competition among the developing progeny in parent generation led to the production of smaller individuals, its influence was not reflected in the reproductive efficiency of the parasitoid when normal conditions were provided, which explains that the parasitoid density in the host caused only the reduction in parasitoid body size but not its reproductive performance.