Akashiwo sanguinea (Hirasaka) G. Hansen et Moestrup was first described in 1922 from water samples collected in Kozusa-ura Gokasho Bay, Japan (Hirasaka 1922). A. sanguinea is cosmopolitan in its distribution and has been observed to form blooms in coastal ecosystems around the world (Kim et al. 1993, Voltolina 1993, Horner et al. 1997, Lee et al. 2001, Lu and Hodgkiss 2004, Feyzioğlu and Öğüt 2006), including the Gulf of Mexico and the Atlantic coast of North America (Robichaux et al. 1998, Steidinger et al. 1998, Badylak and Phlips 2004, Badylak et al. 2007, Phlips et al. 2011, 2012). The cosmopolitan distribution of A. sanguinea is in part attributable to its eurythermal and euryhaline character (Matsubara et al. 2007, Badylak et al. 2014).
Blooms of A. sanguinea have been associated with mass mortalities of invertebrates and fish in various regions of the world (Cardwell et al. 1979, Harper and Guillen 1989, Schumway 1990, Kahru et al. 2004). In the Gulf of Mexico, blooms of A. sanguinea were the suspected cause of fish kills and marine mammal stranding in the mid- 1990’s (Robichaux et al. 1998, Steidinger et al. 1998). One ecologically important feature of dinoflagellate blooms which can contribute to harmful effects is the production of mucous. For example, the smothering of fish by Cochlodinium polykrikoides blooms containing dense concentrations of mucous has been observed in Korea (Kim et al. 2000). Thin horizontal layers of concentrated A. sanguinea densities have been observed in blooms in Monterey Bay, California, USA (Rines et al. 2010). Zooplanktons were found just above and below the thin layer but not within the layer, suggesting that high concentrations of A. sanguinea can negatively influence the distribution of zooplankton.
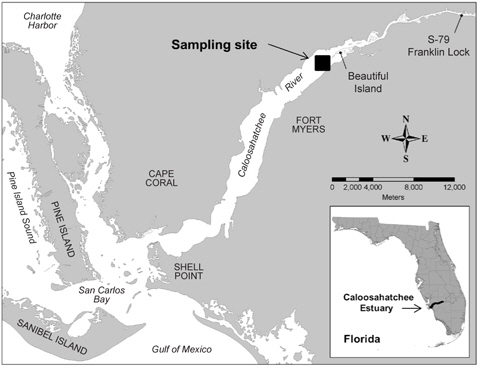
While A. sanguinea has been observed in association with mucous (Voltolina 1993, Robichaux et al. 1998), there have not been clear observations of this phenomenon at the detailed cellular level. In this study we present previously undocumented observations of A. sanguinea extruding mucous substances from pores on the cell surface. The A. sanguinea cells were observed in water samples collected in the Caloosahatchee Estuary, Florida during a bloom event.
A. sanguinea cells observed in this study were from water samples collected in the Caloosahatchee Estuary, Florida during a bloom event. Caloosahatchee Estuary is on the subtropical southwest Florida coastline adjacent to the Gulf of Mexico. The Caloosahatchee Estuary is subject to wide salinity variations due to fresh water releases from Lake Okeechobee, connected to the estuary through a flow-regulated canal.
Salinity measurements were taken with a salinity refractometer (Fisher Scientific, Pittsburgh, PA, USA). Water was collected with a sample pole that allowed the even collection of water from the surface to 0.1 m from the bottom of the water column. Aliquots of well-mixed sample water were preserved with Lugol fixative (i.e., 1 mL concentrate added per 100 mL to yield strong tea color) and stored in an amber bottle for light microscopy and another aliquot was preserved with buffered glutaraldehyde (2% final concentration) for scanning electron microscopy (SEM) observation.
Lugol preserved samples were used to enumerate A. sanguinea cells using the Utermöhl settling method (Utermöhl 1958). In a separate treatment, aliquots of glutaraldehyde preserved water were filtered onto 8 μm pore size nylon membrane filters for SEM analysis, rinsed three times with deionized water, dehydrated in a graded ethanol series and critical point dried (Baltec CPD; Leica Microsystems, Bannockburn, IL, USA). Each filter was placed on aluminum specimen mounts and sputter coated with Au/Pd (Deskll; Denton Vacuum, Moorestown, NJ, USA). High resolution digital micrographs were acquired with a field emission scanning electron microscope (S-4000; Hitachi High Technologies America Inc., Schaumberg, IL, USA).
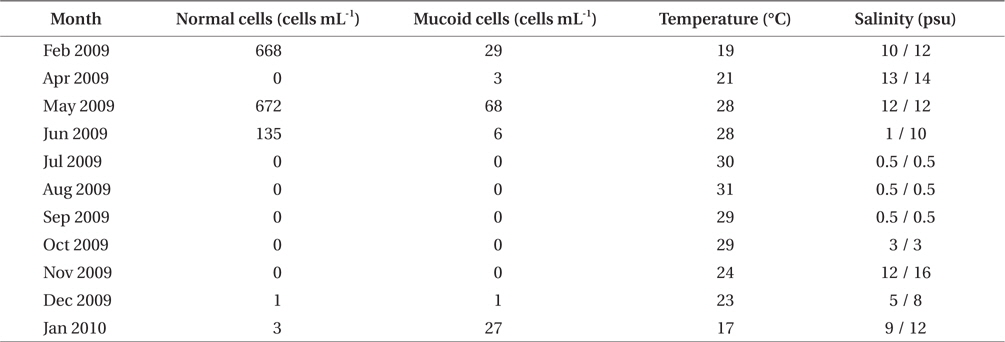
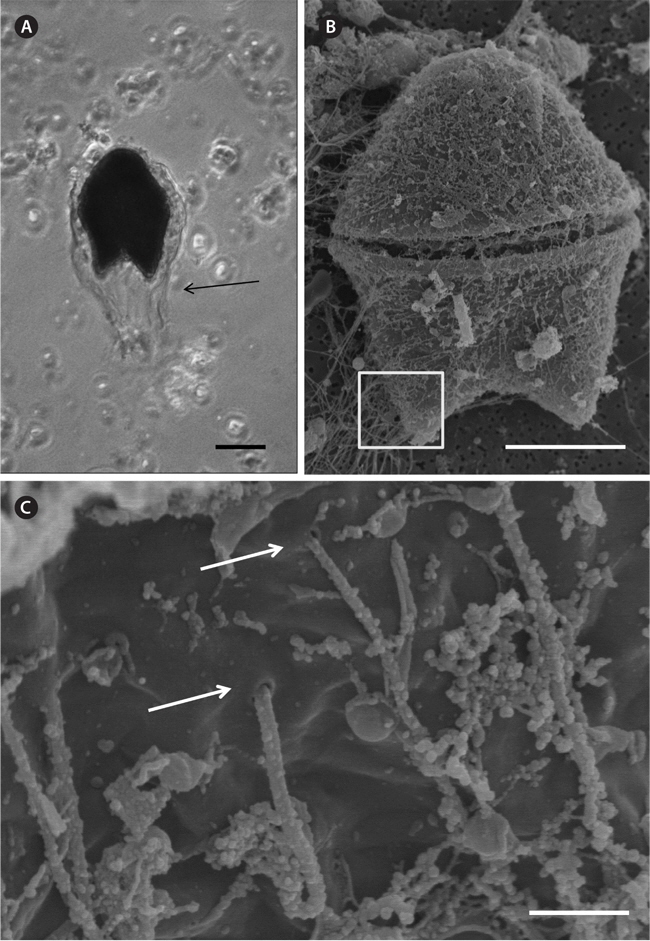
A bloom of A. sanguinea was observed in water samples collected in the upper Caloosahatchee Estuary in February and May of 2009 (Table 1). Salinities during the sample collection were less than as 14 psu. In May A. sanguinea cell densities were 672 cells mL-1. A. sanguinea represented over 90% of total phytoplankton biovolume during the bloom event (Badylak et al. 2014). The sample also contained 68 cells mL-1 of A. sanguinea cells with mucoid substances surrounding the cells (Fig. 1). SEM investigations provided a closer view of A. sanguinea and revealed granular strands of mucous covering the cell surface (Fig. 2A). The SEM images also revealed that the mucous strands were secreted from thecal pores on the cell surface (Fig. 2B). The presence of thecal pores has been observed in other dinoflagellate species and has been linked to the excretion of mucilage (Honsell et al. 2013). It is possible to hypothesize concerning the origin of the mucous strands. One cellular component of dinoflagellates associated with the excretion of extracellular material is extrusomes (Dodge 1973). Another feature is the mucocyst, a type of ejectile organelle found in dinoflagellates that contains fine granular material that can be secreted onto the outer surface of cells (Dodge 1987). There has been no previous description of A. sanguinea possessing mucocysts, but the granular strands extruding from A. sanguinea pores observed in this study could be associated with mucocysts. Transmission electron microscopy is needed to confirm this hypothesis, and to identify the type of mucocyst present, since more than one type of mucocyst exists within dinoflagellates (Zhou and Fritz 1993). It is also possible that the mucous comes from a complex of multiple extrusomes in the cells (Hoppenrath and Leander 2008).
From a functional perspective, excretion of mucilage has been associated with a number of potential benefits, such as dampening turbulence, attachment to benthic substrates, reduction in rates of sedimentation, sequestration of nutrients and resistance to grazing (Smayda 2002, Reynolds 2006). The excretion of mucous has also been linked to the formation of cysts (Reynolds 2006). Resting cysts or hypnozygotes are associated with life cycles and can require a mandatory dormancy period, whereas pellicle or thin walled cysts are a response to adverse conditions (Anderson and Wall 1978, Bravo et al. 2010). The ability to form cysts provides a mechanism for expanding geographical distribution and can act as a defense mechanism to deal with adverse conditions (Smayda 2002).
The highest density of mucous covered A. sanguinea cells was observed in the upper Caloosahatchee, during blooms associated with salinities (i.e., <14 psu) lower than observed in the rest of the estuary during the same time period (Badylak et al. 2014). Salinities during the bloom were on the low end of the range previously reported for A. sanguinea blooms, i.e., 5-30 psu (Matsubara et al. 2007, Badylak et al. 2014). It is possible that the presence of mucoid cells is a response to suboptimal environmental conditions, perhaps a precursor to the formation of resting cysts.
The results of this study demonstrate the excretion of mucilage from thecal pores on the surface of A. sanguinea cells. The observation that A. sanguinea produces mucous provides new insights into the character of the process and possible functional attributes, which may relate to a range of potential benefits, such as protection of cells from adverse conditions and formation of resting cysts.