Algal polysaccharides such as alginate, fucoidan, carrageenan, laminaran, and agar have recently been drawing a great attention from diverse research fields to develop as new biomaterials, health foods, or supplements. Some of these polysaccharides show antitumor (Noda et al. 1990, Lins et al. 2009, Wang et al. 2010), antiviral (Witvrouw and De Clercq 1997, Damonte et al. 2004), anticomplementary (Tissot and Daniel 2003, Clément et al. 2010), anticoagulant (Pereira et al. 1999, Kusaykin et al. 2008), antioxidant (De Souza et al. 2007, Costa et al. 2010), anti-inflammatory (Kang et al. 2011), and immuno- modulatory (Leiro et al. 2007) activities. Brown algae such as
Fucoidans isolated from several algal species including commercially available fucoidan have been reported to possess numerous biological activities such as anticoagulant (Pereira et al. 1999), antithrombotic activities (Kusaykin et al. 2008), antiviral (Karmakar et al. 2010, Sinha et al. 2010), antitumor, anti-inflammatory (Cumashi et al. 2007, Croci et al. 2011), immuno-modulatory (Raghavendran et al. 2011), and apoptosis-inducing activities (Jin et al. 2010, Kim et al. 2010). In addition to these biological activities in mammalian systems, fucoidan was found to be capable of affecting certain bacterial species. It has been reported that fucoidan extracted from
Since only the
Fucoidan isolated from
>
Preparation of methanol-extract and methanolinsoluble fraction of F-fucoidan
Ten mg of F-fucoidan was suspended in 1 mL of methanol, and the mixture was stirred for 24 h at room temperature. Methanol-insoluble fraction and supernatant were separated by centrifugation (2,000 ×g, 5 min). The supernatant (methanol-extract) and the methanol-insoluble fraction were dried up with centrifugal vacuum concentrator (SPD131DDA; Thermo Electron Co., Milford, MA, USA). About 0.68 mg of the methanol extract as dried powder was obtained from 10 mg of F-fucoidan.
RAW264.7 (mouse macrophage) cells obtained from the American Type Culture Collection (Rockville, MD, USA) were cultured at 37℃ in Dulbecco’s modified Eagle’s minimum essential medium (DMEM) supplemented with 10% fetal bovine serum (FBS), penicillin (100 IU mL-1), and streptomycin (100 μg mL-1) in a humidified atmosphere with 5% CO2 and 95% air. Human myeloid leukemia U937 cells obtained from RIKEN Cell Bank (Tsukuba, Japan) were cultured at 37℃ in RPMI1640 medium (Gibco, Gaithersburg, MD, USA) supplemented with 10% FBS in a humidified atmosphere of 5% CO2 and 95% air.
Cytotoxicity of test samples on RAW264.7 cells was measured by MTT tetrazolium cytotoxicity assay. In brief, adherent cells (3 × 104 cells well-1) in a 96-well plate were cultured with varying concentrations of each sample in DMEM for 24 h, and then incubated with MTT (final 10%) for 20 min. After aspiration of the medium, dimethylsulfoxide was added to dissolve the MTT formazan reaction product and the optical density was measured at 535 nm using a multiwell scanning spectrophotometer. Cytotoxicity of test samples on U937 cells was measured by AB assay as described previously (Koyanagi et al. 2003). In brief, 2 × 104 cells per well in a 96-well plate were cultured with varying concentrations of samples in the growth medium for 24 h, and then AB reagent (final 10%) was added to each well. After 2 h incubation at 37℃, the optical density of each well at 570-600 nm was measured with a multiwell scanning spectrophotometer.
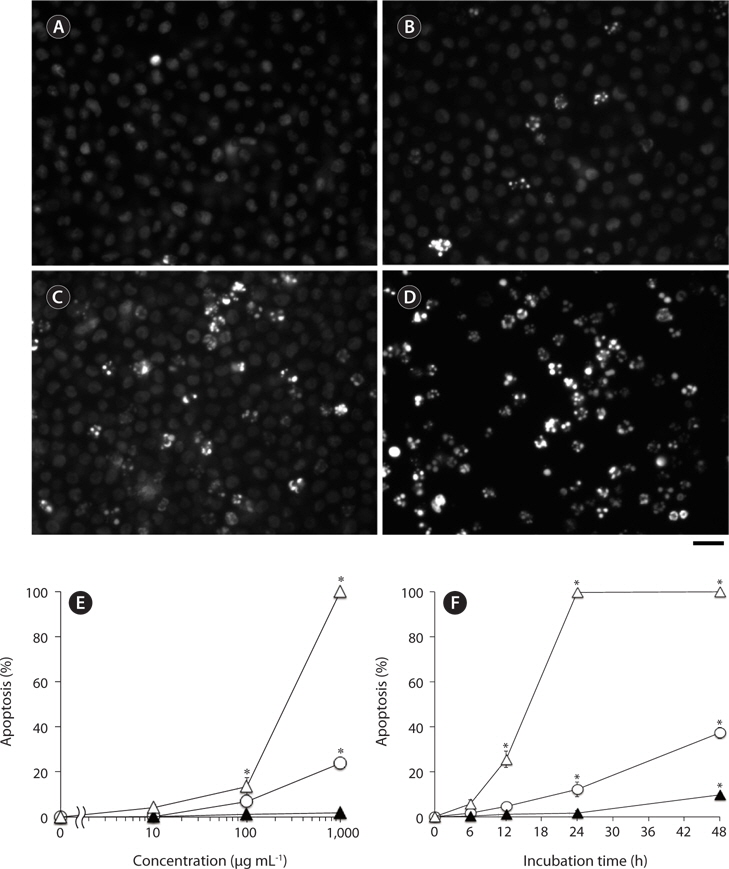
Nuclear morphological changes in U937 cells treated with test samples were examined by staining with DNAbinding fluorochrome bisbenzimide (Hoechst 33342; Dojindo Laboratories, Kumamoto, Japan) as described previously (Nakayasu et al. 2009). In brief, cells in 96-well plates (2 × 105 cells well-1) were incubated with indicated concentrations of each sample in the growth medium at 37℃, and then Hoechst 33342 (final 10 μg mL-1) was added to the treated cells. After 5 min incubation at 37℃, the cells were observed by fluorescence microscope (Axiovert 200; Carl Zeiss, Inc., Jena, Germany). Cells with apoptotic nuclei, i.e., condensed or fragmented, emitting a higher intensity of fluorescence compared with normal cells, were considered as apoptotic cells. At least 100 cells at different areas were observed for each treated group to estimate % of apoptotic cell population.
>
Nitrite assay for the estimation of nitric oxide (NO)
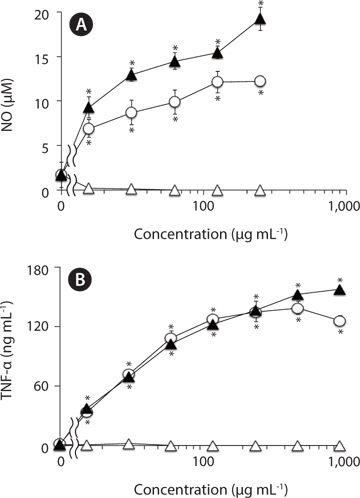
To estimate NO level in RAW264.7 cells, nitrite, a stable reaction product of NO with molecular oxygen, was measured by Griess assay as described previously (Jiang et al. 2011). In brief, adherent RAW264.7 cells in 96-well plates (3 × 104 cells well-l) were incubated with varying concentrations of test samples for 24 h in the growth medium at 37℃, and then the nitrite levels in the culture medium were measured.
>
Enzyme-linked immunosorbent assay (ELISA)
Adherent RAW264.7 cells in 96-well plates (3 × 104 cells well-1) were treated with varying concentrations of test samples in the growth medium at 37℃. After 24 h incubation, the levels of TNF-α in the culture supernatants of treated cells were measured by sandwich ELISA with two antibodies to two different epitopes on TNF-α molecule as described previously (Jiang et al. 2011). The TNF-α concentrations were estimated from a reference to a standard curve for serial three-fold dilution of murine recombinant TNF-α.
For the analysis of carbon, hydrogen, nitrogen, sulfur and oxygen content in the methanol-extract, we used PerkinElmer 2400 Series II CHNS/O elemental analyzer (PerkinElmer, Waltham, MA, USA) according to the manufacture’s instruction. Energy-dispersive X-ray detector (EDS) equipped in scanning electron microscope (JEOL JSM-7500F; JEOL Ltd., Tokyo, Japan) was used for further elemental composition investigation of the methanol-extract.
>
Assay for phenolic compounds
The detection of phenolic compounds in the methanol-extract was carried out by the Folin-Denis method (Niwano et al. 2007). In brief, 3.2 mL of distilled water, 0.2mL of each sample solution or distilled water as a solvent, 0.2 mL of Folin and Ciocalteu’s phenol reagent, and 0.4 mL of saturated sodium carbonate solution were mixed. The absorbance was read at 760 nm after 30 min incubation at room temperature.
All the experiments were repeated at least three times. Data were expressed as means ± standard deviation (SD). Tested groups were compared with appropriate controls using the Student’s t-test. Differences were considered significant at p < 0.01.
>
Antibacterial activities of polysaccharide samples
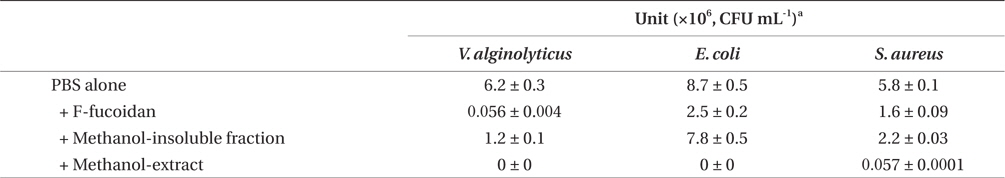
Antibacterial activities of F-fucoidan, A-fucoidan, and ascophyllan were assayed by AB assay using
>
Effects of methanol-extraction on the antibacterial activities of F-fucoidan
Since it has been reported that antibacterial activities were found in organic extracts of
To characterize the antibacterial activities of F-fucoidan, the effects of F-fucoidan on
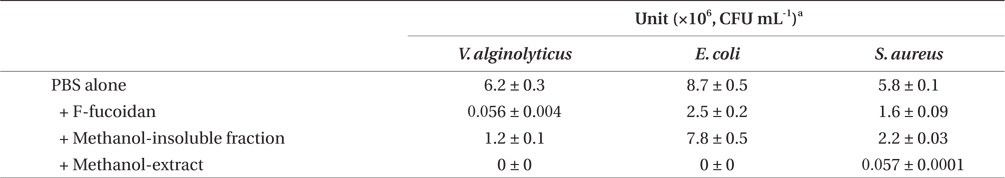
Antibacterial activities of F-fucoidan, the methanol-insoluble fraction, and the methanol-extract on Vibrio alginolyticus, Escherichia coli, and Staphylococcus aureus as measured by colony formation assay
>
Partial characterization of the antibacterial activity of F-fucoidan
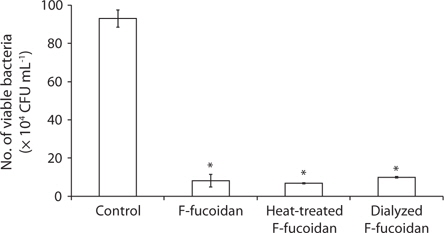
After dialysis of F-fucoidan with molecular porous membrane tube (MWCO; 6,000-8,000) against distilled water for 3 days, the contents inside the tube were lyophilized. As shown in Fig. 2, this simple dialysis of F-fucoidan could not remove the activity. Hence, the antibacterial agents in F-fucoidan may form relatively tight complex with fucoidan molecule through a hydrophobic interaction. After treatment of F-fucoidan at 121℃ for 30 min, no significant reduction of the antibacterial activity was observed (Fig. 2). Furthermore, nearly 99% of antibacterial activity of the methanol-extract passed through the filter that can cut molecules with higher than 3,000 kDa (data not shown). These results suggest that antibacterial agents in F-fucoidan are low molecular weight heat-stable compounds.
>
Chemical composition analysis of the methanol-extract
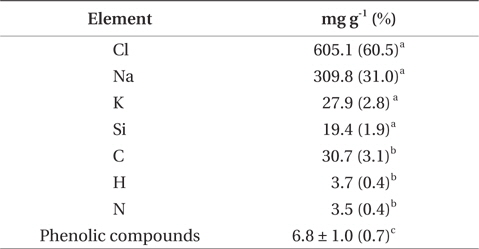
The results of chemical and element analysis of the methanol-extract were summarized in Table 2. Since more than 95% of the methanol-extract were occupied with Na, Cl, K, and Si, the main ingredients of the methanol-extract are considered to be inorganic compounds such as NaCl, and organic compounds were estimated to be only about 3% (w/w). Phenolic compounds in the methanol-extract were detected by Folin-Denis method, and the amount was estimated to be less than 1% of the total weight of the extract.
[Table 2.] Chemical composition analysis of the methanol-extract of F-fucoidan

Chemical composition analysis of the methanol-extract of F-fucoidan
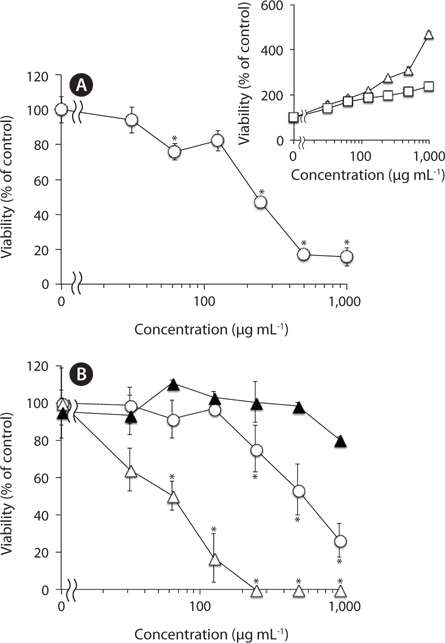
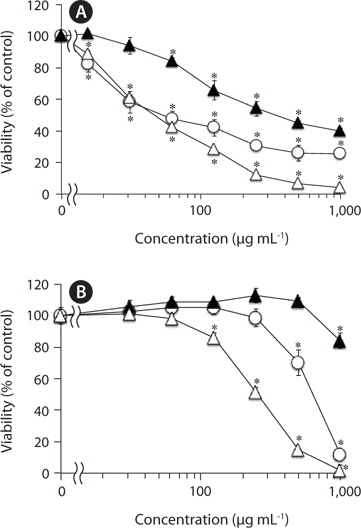
We have previously reported that F-fucoidan, but not A-fucoidan and ascophyllan, showed cytotoxic effect on RAW264.7 cells (Jiang et al. 2011). To investigate the involvement of the methanol-extractable agents of F-fucoidan in the cytotoxicity of F-fucoidan, we examined the cytotoxic effects of the extract and the residual methanol-insoluble fraction of F-fucoidan on RAW264.7 and U937 cells. As shown in Fig. 3A, the methanol-extract showed stronger cytotoxicity on RAW264.7 cells than original F-fucoidan, and the activity of the methanol-insoluble fraction was lower than the original F-fucoidan. The extract also showed a potent cytotoxic effect on U937 cells (Fig. 3B), and nuclear staining revealed that the extract was capable of inducing typical apoptotic nuclear morphological changes in U937 cells in a concentration-dependent manner (Fig. 4A-E). Much greater population of U937 cells treated with the methanol-extract showed such apoptotic nuclear changes than the cells treated with original F-fucoidan. The activity of the methanol-insoluble fraction to induce apoptotic nuclear morphological changes was obviously lower than that of original F-fucoidan, and only a few apoptotic cells were observed in the U937 cells treated with the methanol-insoluble fraction. Time-course analysis of the nuclear morphological changes revealed that the extract induced the apoptotic changes in U937 cells with much earlier time schedule than F-fucoidan, suggesting that underlying apoptosisinducing mechanisms of the extract and F-fucoidan might be different (Fig. 4F).
Fucoidans including commercially available
It has been reported that the fucoidan extracted from
Moreover, the methanol-extract prepared from F-fucoidan exhibit much stronger cytotoxic effects on RAW264.7 and U937 cells than original F-fucoidan, while the cytotoxicities of F-fucoidan decreased after methanol-extraction. Further study showed that the extract was capable of inducing apoptotic nuclear morphological changes in U937 cells. Time-course analysis on the appearances of apoptotic cells showed that the kinetics of the extract to induce apoptosis might differ from that of original F-fucoidan. In other words, F-fucoidan may be contaminated with apoptosis-inducing agents that have a distinct action mechanism from fucoidan itself. The cytotoxicity and apoptosis-inducing activity of F-fucoidan on U937 cells could not completely be eliminated by methanol-extraction.
Partial chemical characterization suggested that the agents responsible for the antibacterial activity in the methanol-extract were low molecular weight heat-stable compounds. Element analysis suggested the methanol-extract contains phenolic compounds in addition to inorganic compounds such as NaCl. It has been reported that phenolic compounds such as phlorotannins present in brown algae showed antibacterial activities against Gram-positive and Gram-negative bacteria with relatively broad antibacterial spectrum (Nagayama et al. 2002). Similar to phlorotannins, the methanol-extract of F-fucoidan showed significant antibacterial activities on
In addition, the methanol-insoluble residual fraction of F-fucoidan exhibited the activities to induce NO and TNF-α from RAW264.7 cells with almost similar concentration- dependent profile to the original F-fucoidan, while the methanol-extract showed no significant activities over the concentration range (0-1,000 μg mL-1) tested, suggesting that the macrophage-stimulating activities of F-fucoidan are mainly attributed to sulfated polysaccharide as a main component.
In conclusion, the results obtained in this study suggested that commercially available