Anthropogenic climate change is rapidly impacting the world’s oceans, increasing sea surface temperatures and seawater acidity (Feely et al. 2004, Intergovernmental Panel on Climate Change 2007, Doney et al. 2014). Over the last century, rising atmospheric CO2 levels have caused a 1℃ increase in sea surface temperatures, causing shifts in species ranges and changes in community assemblages (Poloczanska et al. 2013, Burrows et al. 2014). In addition to ocean warming, climate change is also altering the chemistry of the ocean through the process of ocean acidification, causing a 0.1 unit decrease in average ocean pH over the past century and a shift in the distribution of carbon speciation by decreasing levels of carbonate (CO32-) and increasing levels of free CO2 (Feely eleet al. 2004, Doney et al. 2009). Predictions for the end of the century, based on various anthropogenic emission scenarios, estimate further increases in average sea surface temperature and ocean pCO2, with high emission scenarios resulting in increases of 3-4℃ and ~500 μatm CO2, respectively (Intergovernmental Panel on Climate Change 2007, Doney et al. 2014). At these pCO2 levels, decreases in ocean pH of 0.3-0.4 units are predicted (Caldeira and Wickett 2003) with the potential to negatively impact marine species on a global scale.
As increases in ocean temperature, pCO2 and acidity are expected to have far reaching impacts on ocean life, over the last decade there has been a near exponential increase in the number of studies examining the impacts of ocean climate change on marine organisms across a wide range of taxonomic groups (see Wernberg et al. 2012, for review). Despite our growing understanding of how climate change will impact marine species, making accurate predictions of how marine ecosystems will respond remains difficult for several reasons. Firstly, the predicted increases in seawater temperature and pCO2 (Intergovernmental Panel on Climate Change 2007) are based on models for the open-ocean where values are expected to track atmospheric concentrations, and therefore they may not apply to coastal ecosystems where these values are much more dynamic (e.g., Hofmann et al. 2011). Secondly, despite the fact that most marine organisms will be exposed to concurrent increases in temperature and pCO2 as the climate changes, relatively few studies (i.e., less than 10% between 2000 and 2010) (Wernberg et al. 2012) have examined the impacts of simultaneous increases in both temperature and pCO2. Consequently, such single factor studies ignore possible interactive effects between temperature and pCO2 (Crain et al. 2008), making it difficult to predict how climate change will ultimately impact marine organisms. Finally, the majority of studies have focused on either the physiological or demographic impacts on single species and have largely ignored how climate change will impact species interactions. Changes in such interactions may aggravate or ameliorate the impacts of environmental stressors on an organism (O’Connor 2009, Falkenberg et al. 2013), ultimately resulting in outcomes different from what studies on single species would predict. As a result, care must be taken when extrapolating the physiological effects on single species to community-level impacts on the ecosystem as a whole. This is especially important for complex ecosystems such as coastal kelp forests where community structure is strongly influenced by species interactions.
Kelp forests are among the most productive ecosys204tems in the world. Along the southern coast of California, USA, pH values within forests can vary by 0.2-0.4 pH units over the course of a single day (Hofmann et al. 2011), exposing organisms within the forest to lower pH values compared to organisms in less variable environments like the open ocean. The dominant canopy-forming kelp along the California coast is the giant kelp, Macrocystis pyrifera. This species grows at depths up to 30 m and can reach up to 50 m in length (Dayton 1985). Its main thallus forms a large three dimensional structure that provides food and habitat for numerous species of invertebrates, fish and marine mammals, some of which are exclusively associated with kelp forests (Dayton 1985). Many kelp forests are notable in that they tend to be dominated by a single type of herbivore, stronglyocentroid sea urchins, and the structure of kelp forest communities is often determined by the interaction between sea urchins and kelp (Harrold and Reed 1985). Sea urchins are generally sedentary foragers which subsist on drift kelp (Dean et al. 1984, Harrold and Reed 1985), but when large numbers begin to actively forage they can denude large swaths of kelp from the benthos (Estes and Palmisano 1974, Harrold and Reed 1985), creating urchin barrens which may persist for years or decades (Mann 1977, Leinaas and Christie 1996, Konar et al. 2014). Urchins within barren grounds often show signs of food limitation such as smaller gonads (Dean et al. 1984, Harrold and Reed 1985), suggesting that changes in the quantity or quality of their food supply may be instrumental in their foraging behavior, and therefore the formation of barrens. Along the coast of California, one the most abundant species of urchin is the purple sea urchin Strongylocentrotus purpuratus, which consumes a wide variety of algae, including kelp (Kenner 1992). Given that the interaction between S. purpuratus and M. pyrifera can play an important role in structuring kelp forest communities, how these organisms respond to climate change could have serious consequences for coastal ecosystems.
Like most organisms with calcium carbonate skeletons, sea urchins are expected to be vulnerable to ocean acidification due to decreases in CO32- concentrations and higher carbonate dissolution rates in more acidic waters (Doney et al. 2009). However, the degree to which they are vulnerable is equivocal. Studies on adult urchins under elevated pCO2 have shown negative impacts to internal acid / base balances (Catarino et al. 2012), and increases in metabolic rates (Spicer et al. 2011), but the effects of pCO2 on calcification may be species dependent, with some species actually showing increases in calcification (Ries et al. 2009). In addition, it is unclear whether elevated temperature will ameliorate the negative effects of elevated pCO2 (Brennand et al. 2009), or make organisms more vulnerable to the negative effects of acidification (Pörtner 2008). While climate change studies on marine invertebrates have become increasingly common, relatively few studies have examined the effects of climate change on macroalgae (Wernberg et al. 2012), with those that have focusing largely on calcifying algae (Koch et al. 2013). Consequently, in spite of their importance as ecosystem engineers, the effects of climate change on non-calcifying macroalgae, are relatively understudied. Although the majority of marine macroalgae, including M. pyrifera (Parker 1965), are capable of utilizing HCO3- in photosynthesis through the use of carbon concentrating mechanisms, many macroalgae appear to be carbon limited under present-day pCO2 conditions (Koch et al. 2013) and may therefore benefit from CO2 enrichment. However, short-term increases in photosynthesis from increased CO2 concentrations do not always result in increased growth rates over longer periods (Israel and Hophy 2002). Likewise, while elevated temperature often causes shortterm increases in macroalgal photosynthetic rates (Davison 1987), longer-term incubations have shown the opposite effect, with lower rates of photosynthesis under higher temperatures (Davison et al. 1991). For M. pyrifera in particular, only two recent studies (Roleda et al. 2012, Gaitán-Espitia et al. 2014) have examined the physiological effects of elevated temperature and pCO2, with both studies focusing on the microscopic life stages. Given these ambiguities, it is difficult to predict with certainty how S. purpuratus and M. pyrifera will respond to elevated temperature and pCO2, and it is unclear what impacts their responses will have for kelp forest ecosystems.
This study attempts to determine how elevated temperature and pCO2 will affect adult S. purpuratus and the adult sporophyte of M. pyrifera, and whether these factors will impact the interaction between these species. To determine how M. pyrifera will respond physiologically to increases in pCO2 and temperature, we examined the individual and combined effects of elevated pCO2 and temperature on M. pyrifera growth and photosynthetic performance. In a second experiment, the effects of elevated temperature and pCO2 on growth, feeding and gonad index of S. purpuratus were observed, with attention on decoupling the direct impacts of climate change on the urchins themselves from the indirect effects on urchins caused by eating kelps also subjected to climate change conditions.
In August 2012, meristematic tissues were collected from 72 randomly selected M. pyrifera sporophytes at a depth of 12-15 m near the center of the Point Loma kelp forest, San Diego, CA using SCUBA. The meristems were brought to the surface in the dark, immediately placed in a cooler filled with seawater, and transported to San Diego State’s Coastal Marine Institute Laboratory. Meristems were trimmed to a wet weight of ~7 g and placed into one of four 90 L acrylic mesocosms (n = 18 per mesocosm). Each mesocosm was connected to one of four 100 gallon holding tanks, filled with seawater collected from a nearby site at the beginning of the experiment. Seawater temperature and pCO2 levels within each holding tank were adjusted to make four orthogonal treatment combinations; a “present-day” treatment (12℃ and 500 μatm pCO2), an “elevated temperature” treatment (15℃ and 500 μatm pCO2), an “elevated CO2” treatment (12℃ and 1,500 μatm pCO2), and a “future” treatment at (15℃ and 1,500 μatm pCO2). It must be noted that logistic constraints associated with our seawater system required that all individuals for each treatment to be cultured within single mesocosms (i.e., mesocosms and seawater holding tanks could not be independently replicated). Despite the well-known problems of interpreting data based on pseudoreplicated samples (Hurlbert 1984), we point out that this is not uncommon in climate change mesocosm studies (Wernberg et al. 2012) or studies that use laboratory growth chambers, which hold other environmental parameters constant. However, we caution the reader with this caveat.
The “present-day” conditions were selected to approximate average ambient temperature and pCO2 levels within the Point Loma kelp forest during the experiment (Edwards and Kim unpublished data), while the levels for the experimental treatments were selected based on predicted increases in temperature and pCO2 for the end of the 21st century (Intergovernmental Panel on Climate Change 2007). pCO2 levels within the holding tanks were maintained by bubbling either ambient air or a certified air / CO2 mixture (1,485 ppm pCO2, Praxair San Diego) into the tanks using Venturri injectors. To ensure pCO2 levels remained at the levels desired, water samples were taken from the outflow lines of the holding tanks on each day and analyzed using potentiometric titration to determine total alkalinity (TA) and total inorganic carbon (TIC) within the tank water. These values were then used to calculate the pCO2 within the tanks using the program CO2SYS (http://cdiac.ornl.gov/ftp/co2sys/). pCO2 levels within tanks varied over the course of the experiment (see results), but remained within the range of natural variability experienced within the Point Loma kelp forest (Edwards and Kim unpublished data). Temperature in the tanks was maintained at either 12 or 15℃, depending on treatment, using aquarium chillers connected to a control box that constantly monitored temperature within the tanks. Light above the mesocosms was provided by full spectrum fluorescent bulbs set on a 12 : 12 cycle, with light levels set at 15-20 μmol photons m-2 s-1, approximately the same levels the kelps experienced at the depths where they were collected (M. Edwards unpublished data). To ensure algae were not nutrient limited during the experiment, nitrate within the water was measured every 3-4 days using a Lachat QuickChem 8000 FIA (Lachat Inc., Loveland, CO, USA) and used as a proxy for the total nutrient profile within the holding tanks. If nitrate levels were found to be lower than 1-2 μM, 1-2 mL of Proline algae fertilizer (Guillards; Pentair, Sanford, NC, USA) was added to the holding tanks.
M. pyrifera meristematic tissues were held within the mesocosms for one month. To determine if M. pyrifera growth rates differed between the four seawater treatments, wet weights of the meristems were measured weekly, and growth rates were determined as the change in average meristem weight within each treatment over the course of the experiment. To determine carbon to nitrogen (C : N) ratios of meristems, approximately 1 g was excised from the most basal blade of each meristem in each treatment. These blade sections were dried overnight in an oven and then ground to a fine powder using a Wiley Mill plant grinder (Thomas Scientific, Swedesboro, NJ, USA). Total carbon and total nitrogen content of the tissue samples were then measured using a ECS 410 Elemental Analyzer (Costech Analytical, Valencia, CA, USA) and the ratio calculated as % carbon / % nitrogen.
To determine the effects of elevated temperature and pCO2 on M. pyrifera, photosynthesis, photosynthetic carbon uptake by the meristems was measured at the end of the one-month experiment. Each treatment was measured separately over four consecutive days. On each day, eighteen 500 mL BOD bottles were filled with seawater taken from the treatment being measured, sealed and stored in darkness within a temperature-controlled room, set to the temperature of the mesocosm treatment. A separate water sample was taken from the holding tanks to determine starting TIC for each treatment. Concurrently, blade sections (~1 g wet weight) were taken from the basal blade of three separate M. pyrifera meristems in the treatment being evaluated. Each blade section was patted dry, weighed and placed into a BOD bottle. These bottles were then placed upright in separate water baths within the temperature-controlled room, and photosynthetic carbon uptake by the blades was measured under seven irradiances (0, 5, 10, 15, 30, 75, and 125 μmol photons m-2 s-1) for one hour each. Light was provided by full-spectrum compact fluorescent bulbs hung directly above the bottles and light levels were adjusted either by moving the bulb closer to the bottles, or through window tinting of different shading properties. To reduce the formation of boundary layers, magnetic stirring bars were placed into each bottle. After one hour, the blade sections were removed and the TIC within the bottles measured using potentiometric titration as described above. Carbon uptake was calculated as the difference between TIC at the end of one hour versus the treatment’s starting TIC and standardized to the weight of the blade section. The blade sections were transferred to new bottles and the irradiance was increased to the next level. For each treatment the carbon uptake at each light level was plotted and fit using Platt et al. (1975). From these best-fit lines, the maximum rate of photosynthesis (Pmax) and the photosynthetic efficiency (α) of each treatment were calculated.
To determine how temperature and CO2 will impact S. purpuratus both directly and indirectly through induced changes in M. pyrifera, a three-month feeding study was performed from January to March 2013. The four 100-gallon holding tanks (described above) were refilled with new seawater and assigned to one of two water treatments; “present-day” conditions (12℃ and 500 μatm pCO2) and “future” conditions (15℃ and 1,500 μatm pCO2). Seawater within the holding tanks was replaced one month into the experiment and every two weeks after that, with the temperature and pCO2 levels within the holding tanks adjusted in the manner described above. Each holding tank was connected to two mesocoms, one large (90 L) and one small (18.5 L), for a total of eight mesocosms. M. pyrifera were held in the large mesocosms under 15-20 μmols m-2s-1 photons irradiance, while S. purpuratus were held in the small mesocosms out of direct light.
Thirty-six purple urchins, Stronglyocentrotus purpuratus (Stimpson), with test diameters between 3 and 5 cm were collected and transported in the manner described above. Urchins were starved within a holding tank for three weeks, after which they were randomly assigned to one of four groups of nine, with each group assigned to one of the four small mesocosms. Each small mesocosm was divided into nine, equally sized compartments using Vexar mesh, with each individual compartment holding a single urchin. The initial total weights were recorded for each urchin to the nearest 0.01 g (initial average weights below). One week before the urchins were placed into the small mesocosms, meristems of M. pyrifera were collected from the Point Loma kelp forest and brought to the laboratory in the manner described in the previous experiments. Approximately 14 meristems were placed into each of the large mesocosms, creating four groups of kelp within two water treatments (present-day and future). Meristems were allowed to acclimate to their new water conditions for one week before being fed to the urchins. Once feeding had begun, new meristems were collected weekly and acclimated to their treatment conditions as described above before being fed to the urchins. Although effort was made to allow all meristems to acclimate for one week prior to feeding, occasionally poor weather conditions prevented dive operations and the meristems were acclimated for less than a week. To determine how temperature and pCO2 induced changes in M. pyrifera impact S. purpuratus growth, the urchin groups were assigned one of two feeding regimens. One “present-day” group and one “future” group were fed kelp meristems from their own respective water treatments (e.g., urchins held under present-day conditions were fed kelps acclimated to present-day conditions), while the second “present-day” and “future” groups were fed kelp from the opposite water treatment (e.g., urchins held under present-day conditions were fed kelps acclimated to future conditions). This combination resulted in four final treatments; present-day urchins fed present-day kelp (hereafter PUPK, average weight = 45.14 g); present-day urchins fed future kelp (PUFK, average weight = 41.5 g); future urchins fed present-day kelp (FUPK, average weight = 43.65 g); and future urchins fed future kelp (FUFK, average weight = 42.75 g).
Urchins were fed continuously throughout the experiment, with the exception of one three-week interruption due to inclement weather which made collecting kelp impossible, approximately six weeks after the experiment began. Each day, roughly equal sections were cut from the blades of a single meristem, weighed and placed into each individual urchin compartment. After 24 hours, any remaining pieces of the blade sections were removed and the urchins were given new blade sections from a new meristem, with selection of the meristems alternating between the large mesocosms for that water treatment. Urchin feces were siphoned out of the small mesocosms daily to prevent both their accumulation and the buildup of nitrogenous waste in the water.
Feeding rates for each urchin group were measured on three occasions: one month after the start of the experiment, one day after the aforementioned three-week interruption, and two weeks after the interruption. On the days feeding rates were measured, urchins and blade sections were weighed before feeding commenced. At the end of 24 hours, any remaining blade pieces were removed and reweighed. Feeding rates were calculated as the difference in blade weight before and after the feeding period standardized by urchin weight. At the end of the study, the ending test size and total weight of each urchin was measured in the manner described above. Urchins were then dissected and their gonads, somatic tissues and tests weighed. From these measurements each urchin’s gonad index was calculated as gonad weight / total weight × 100.
All data met assumptions of normality and homoscedasticity as determined by visual inspection of the residuals and Levene’s test, respectively. M. pyrifera growth rates, carbon uptake, and C : N ratios were compared among the different temperatures and pCO2 levels using separate two-factor Model I ANOVA’s. Similarly, S. purpuratus growth rates, feeding rates and gonad indices were compared among the urchin treatments (“present-day” vs. “future”) and kelp treatments (“present-day” vs. “future”) using separate two-factor Model I ANOVA’s.
Seawater pCO2 and temperature within the 100-gallon holding tanks remained within the desired ranges during the experiment. Specifically, seawater temperature did not vary from their desired levels by more than 1℃ on any day during the course of the experiment, and pCO2 within the two 500 μatm pCO2 treatments (12 and 15℃) ranged between 400-600 μatm pCO2, while levels in the two 1,500 μatm pCO2 treatments ranged between 1,200-1,500 μatm pCO2 (data not shown).
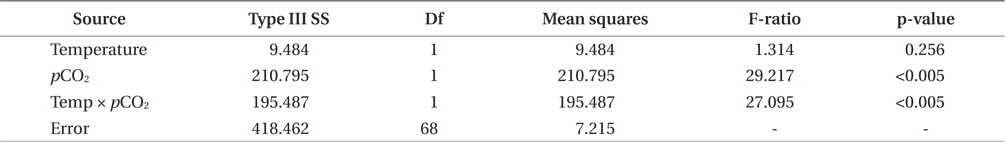
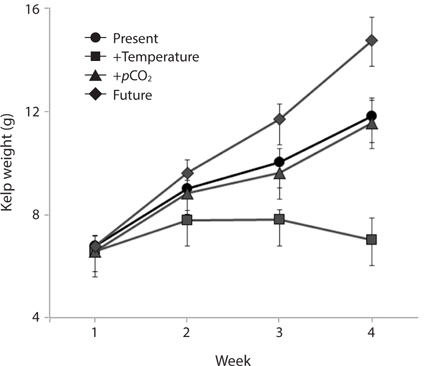
Growth rates of the M. pyrifera meristems within the experimental mesocosms varied significantly between the pCO2 treatments (ANOVA: F1,58 = 29.21, p < 0.001) but not between the two temperatures (F1,58 = 1.31, p = 0.25). However, these two factors interacted such that the effect of pCO2 depended on temperature (pCO2 × temperature interaction: F1,58 = 27.09, p < 0.001) (Table 1). Specifically, meristems within the elevated temperature treatment exhibited the lowest growth rates of all the treatments and by the end of the one-month experiment, these meristems showed biomass loss due to tissue deterioration (Fig. 1). In contrast, growth was observed in all other treatments, with growth in the “future” treatment being the highest and resulting in a doubling of the meristem weights (Fig. 1). Growth in both the “present-day” and elevated pCO2 treatments were intermediate and did not differ significantly from each other (Fig. 1).
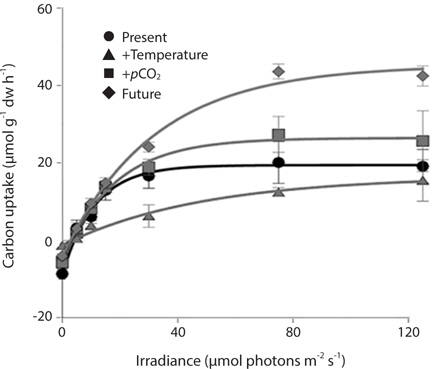
Photosynthetic performance of M. pyrifera meristems, measured by carbon uptake, varied among the different seawater treatments (Fig. 2). Specifically, meristems held under future conditions exhibited the highest maximum rates of photosynthesis (Pmax), more than twice those observed under the “present-day” conditions (ANOVA: F1,12 = 7.687, p = 0.018). Similar to growth rate, Pmax was significantly greater under future conditions than under present-day (Tukey’s: p = 0.016), elevated pCO2 (p = 0.045) or elevated temperature conditions (p = 0.002). In contrast, Pmax did not vary significantly among M. pyrifera meristems held under present-day conditions versus conditions where only pCO2 was elevated (p = 0.56). Likewise, Pmax also did not vary among meristems grown under present-day and elevated temperature conditions (p = 0.11). In contrast, Pmax varied significantly among meristems held under elevated pCO2 compared to those held under elevated temperature (p = 0.04). Photosynthetic efficiency (α) also varied significantly between seawater treatments (ANOVA: F1,12 = 12.31, p < 0.05). Under non-saturating irradiances, α did not differ between meristems held under present-day and elevated CO2 conditions (p = 0.21). However, α in meristems held under future conditions was significantly lower than in meristems held under present-day conditions (p = 0.05), while α in meristems held under elevated temperature conditions was significantly lower than under present-day (p = 0.002), elevated pCO2 (p = 0.011) or future (p = 0.039) conditions. These findings show that changes in temperature and pCO2 interact to influence carbon uptake in M. pyrifera, with the negative effects of elevated temperature being ameliorated by higher pCO2.
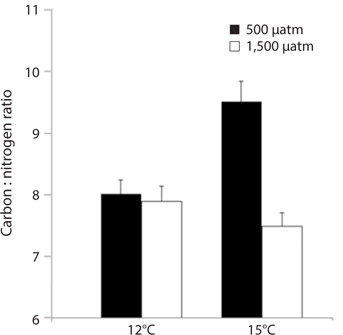
M. pyrifera tissue C : N ratios varied significantly between present-day and future pCO2 conditions (ANOVA: F1,45 = 10.164, p = 0.003) but not between the two temperature treatments (F1,45 = 2.67, p = 0.109). However, pCO2 and temperature interacted such that the overall effect of elevated pCO2 depended on the temperature (temperature × pCO2 interaction, F1,45 = 7.996, p = 0.007). Specifically, C : N ratios in the meristem tissues did not differ significantly between M. pyrifera held under present-day, elevated pCO2 conditions, or future conditions, but did exhibit significantly higher C : N ratios under elevated temperature compared to present-day (Tukey’s: p = 0.05), elevated pCO2 (p = 0.04), and future conditions (p = 0.005) (Fig. 3). This difference was primarily due to elevated carbon content as no significant difference in nitrogen content was observed (data not shown).
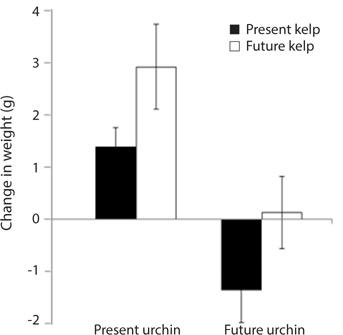
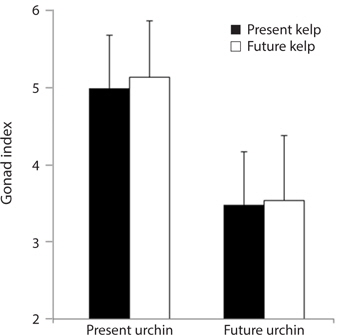
Growth rate and gonad index. Growth rates of S. purpuratus differed significantly between individuals held under present-day and future conditions (ANOVA: F1, 24 = 14.79, p = 0.001). Specifically, S. purpuratus raised under present-day conditions grew significantly more than urchins held under future conditions regardless of the conditions under which their food was held (Fig. 4). In contrast, while urchins held under present-day conditions showed higher total weight at the end of the experiment, urchins under future conditions showed either no change in total weight or decreases in weight by the end of three months. Further, the conditions under which their food (M. pyrifera) was grown also had a significant effect on urchin growth, with the urchins feeding on M. pyrifera held under future conditions growing significantly more rapidly than urchins feeding on M. pyrifera held under present-day conditions (ANOVA: F1,24 = 4.39, p = 0.047) regardless of the conditions under which the urchin were held (ANOVA: urchin × kelp, F1,24 = 0.001, p = 0.98). In addition, urchin gonad indices were significantly impacted by the conditions under which the urchins were held, with higher indices observed in urchins held under present-day conditions than urchins held under future conditions (ANOVA: F1,24 = 3.243, p = 0.09) (Fig. 5). In contrast to the total growth weight of the urchins, no effect of the conditions under which food was grown was observed on urchin gonad size (ANOVA: F1,24 = 0.057, p = 0.831). In addition, there was no significant interaction between urchin treatment and kelp treatment on gonad size (ANOVA: F1,24 = 0.085, p = 0.76).
[Fig. 4.] Mean growth (changes in total weight, ± standard error) of Strongylocentrotus purpuratus held under present-day and future temperature and pCO2 conditions. Left-hand bars represent S. purpuratus held under present-day conditions (i.e., 12℃ and 500 μatm pCO2) and fed kelp held under both present-day (PUPK) and future (PUFK) (i.e., 15℃ and 1,500 μatm pCO2) conditions. Right-hand bars represent S. purpuratus held under future conditions and fed kelp held under both present-day (FUPK) and future (FUFK) conditions. Urchins were weighed at the beginning and end of the three-month study. Sample size for PUPK, PUFK, FUPK, and FUFK is n = 9, n = 8, n = 7, and n = 5, respectively.
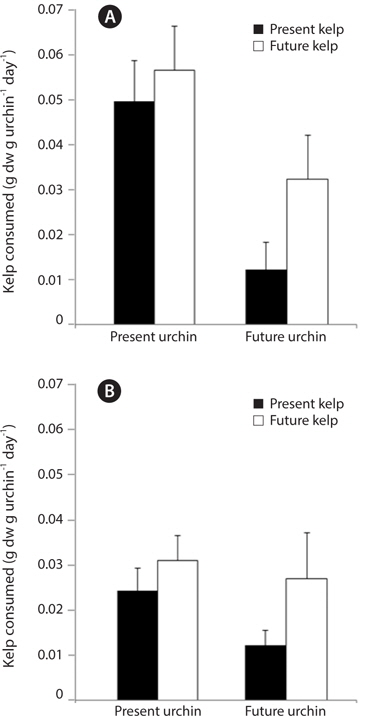
In the first feeding trial, urchin condition had a significant effect on urchin feeding rate (ANOVA: F1,32 = 10.29, p = 0.003), with urchins held under present-day conditions exhibiting significantly greater feeding rates than urchins held under future conditions (Fig. 6A). In contrast, and similar to patterns observed in urchin gonads, the conditions under which their food, M. pyrifera, was held did not impact urchin feeding rates (F1,32 = 2.41, p = 0.13), although there was a non-significant increase in feeding rates among urchins fed kelp from future conditions. The effect of urchin conditions on feeding rate did not vary depending on the conditions kelp were raised under (urchin × kelp interaction, F1,32 = 0.35, p = 0.55). In the second feeding trial, none of the factors had a significant impact on feeding rate (ANOVA: urchin, F1,32 = 0.19, p = 0.66; kelp, F1,32 = 0.29, p = 0.59; urchin × kelp, F1,32 = 0.49, p = 0.48). This feeding trial took place immediately following a three-week starvation, and all urchin groups consumed amounts of kelp (roughly 0.6 g per g urchin on average) comparable to the highest feeding rates seen in the first feeding trial (data not shown). Similarly, the third feeding experiment revealed no effects of either the conditions under which the urchin were held (ANOVA: F1,26 = 1.66, p = 0.20), or the conditions under which their food was grown (F1,26 = 2.90, p = 0.10) on S. purpuratus feeding rate, nor did these factors interact (urchin × kelp, F1,26 = 0.40, p = 0.53) (Fig. 6B). Interestingly, feeding rates of urchin groups in the third trial were generally lower compared to the first two feeding trials. Consequently, while the conditions under which M. pyrifera is grown may impact urchin feeding rates, these patterns can be weak and often not significant. However, one trend that was observed is that urchins held under future conditions may exhibit reduced feeding, especially when feeding on kelps grown under future conditions.
[Fig. 6.] Feeding rates, measured as the amount of kelp consumed g urchin-1 day -1, of sea urchins at one month (A) and two months (B) after the start of the three month urchin incubation. Kelp pieces were weighed prior to feeding and placed into individual urchin compartments. Urchins were allowed to feed for 24 hours, after which any remaining kelp was removed and reweighed. Data are means + standard error. n = 9 for all treatments in (A). In (B), n for PUPK, PUFK, FUPK, and FUFK was n = 9, n = 8, n = 8, and n = 5, respectively.
As anthropogenic emissions of greenhouse gases continue to increase, the world’s oceans will progressively become warmer and more acidic (Caldeira and Wickett 2003, Intergovernmental Panel on Climate Change 2007). Marine organisms will not only have to adapt to these concomitant increases in temperature, acidity and pCO2, but also to changes in biotic interactions as climate change alters community structure and organism physiology / behavior. To date, the majority of climate change studies have focused on the individual effects of temperature and / or pCO2 on single species (reviewed in Wernberg et al. 2012), but using these studies to make predictions of how ecosystems will respond to future climate change is problematic. One reason for this is that environmental stressors, such as increases in temperature and / or pCO2, may not support the same relationships in all circumstances, but instead may interact in complex ways depending on their relative strength and on the organisms in which they are examined (Crain et al. 2008). In addition, the direct effects of these stressors on organism physiology may be altered by changes in the strength of species interactions (O’Connor 2009, Falkenberg et al. 2013), resulting in outcomes different than those observed when species are studied in isolation. In this study, we demonstrated that the physiological responses of the giant kelp, M. pyrifera, to predicted increases in temperature and pCO2 (Intergovernmental Panel on Climate Change 2007) vary greatly between situations where these factors are examined individually versus together. In addition, while increases in temperature and pCO2 negatively affected growth and feeding in the purple sea urchin, S. purpuratus, these effects were at least partially ameliorated by the effects of increased temperature and pCO2 on the M. pyrifera on which the urchins feed. Whether kelp or sea urchins will respond similarly under natural settings is unclear, but these data do highlight the importance of conducting ecologically realistic studies of climate change that consider the interactive effects of multiple stressors and species interactions, and therefore have important implications for the future of coastal kelp forest ecosystems.
Despite its worldwide distribution and its status as an important habitat ecosystem engineer (Dayton 1985), relatively little is known about how M. pyrifera will respond to a warmer, more acidic ocean (reviewed in Harley et al. 2012), particularly the adult sporophyte stage. In the first experiment, M. pyrifera meristems held under elevated pCO2 alone showed little to no changes in growth rate, carbon uptake, or tissue C : N ratio relative to meristems held under present-day conditions, suggesting that M. pyrifera is carbon-saturated under current pCO2 levels. In contrast, M. pyrifera growth, carbon uptake, and tissue C : N ratios were strongly impacted by elevated temperature. Specifically, M. pyrifera held under elevated temperature alone exhibited reduced growth, higher C : N ratio, lower Pmax, and reduced photosynthetic efficiency (α) relative to meristems held in other treatments. In addition, by the end of the experiment, significant tissue deterioration was observed in the M. pyrifera held under elevated temperature, compared to other treatments where M. pyrifera continued to grow throughout the experiment. Perhaps most interesting is that M. pyrifera held under conditions where both temperature and pCO2 were increased together (i.e., predicted future conditions) exhibited the greatest growth and highest rates of carbon uptake during photosynthesis. In fact, Pmax in M. pyrifera held under future conditions was nearly double that of M. pyrifera held under present-day conditions. This may have been due to the effect of temperature on RuBisCO activity during photosynthetic carbon reduction (i.e., the Calvin cycle). Higher temperatures are known to increase the reaction rate of RuBisCO (Davison 1987) and, at least under present-day pCO2 conditions, to increase oxygenase activity relative to carboxylase activity (Jordan and Ogren 1984). Thus, it is possible that M. pyrifera raised under elevated temperatures suffered losses of photosynthetic carbon reduction due to higher rates of photorespiration. In contrast, M. pyrifera raised under higher temperature and pCO2 concentrations together presumably exhibited alone, due to the saturation of RuBisCO’s active sites with CO2. Furthermore, the increased reaction rates induced by higher temperatures may have allowed M. pyrifera to take advantage of the increased CO2 concentrations by increasing the rate of CO2 binding, thus facilitating the higher rates of growth and carbon uptake observed. diminished oxygenase activity compared to highpCO2
In addition to altering organism physiology, environmental stressors can alter the manner in which species interact (O’Connor 2009, Falkenberg et al. 2013). S. purpuratus held under both elevated temperature and pCO2 (i.e., future conditions) exhibited significantly lower growth rates, reduced feeding rates, and smaller gonad indices than urchins held under present-day conditions. Although it is unclear which factor, temperature or pCO2, was responsible for these differences, metabolic rates are known to increase with temperature across a wide range of organisms (Gillooly et al. 2001), and increased metabolic costs associated with elevated pCO2 have been observed in sea urchins (Spicer et al. 2011, Catarino et al. 2012). Thus, the decrease in growth rate may be due to changes in physiology brought on by increases in either temperature or pCO2. Physiological changes, such as increased metabolism, might also explain the lower gonad indices in the urchins held under future conditions, as higher metabolic costs would leave less energy available for gonad production and energy storage. Supporting this idea, other studies have observed decreases in gonad size under elevated pCO2 (Siikavuopio et al. 2007, unpublished data referenced in Kurihara 2008). An alternative explanation, though obviously not mutually exclusive, is that the lower growth rates of urchins held under future conditions are due to lower feeding rates in those urchins. Feeding rates, like other processes tied to metabolism, are generally thought to increase with increasing temperature (Brown et al. 2004), but elevated pCO2 has been shown to decrease feeding rates in sea urchins (Siikavuopio et al. 2007). Perhaps the most interestingly, the direct effects of elevated temperature and pCO2 on the urchins were, at least partially, ameliorated by changes in their diets, with M. pyrifera raised in future conditions allowing significantly higher growth and feeding rates in the urchins that fed on them. Changes in feeding rate may be caused by changes in food quality, and studies have shown correlations between changes in C : N ratio, a good metric of nutritional value, and either increases (Stiling and Cornelissen 2007, Falkenberg et al. 2013) or decreases (Cruz-Rivera and Hay 2000) in feeding rate. However, analysis of M. pyrifera tissue C : N ratios showed no significant difference between individuals raised under present-day and future conditions. As a final caveat, it must be noted that the future urchin / future kelp treatment exhibited significantly higher rates of mortality compared to urchins in other treatments. The cause behind this is unclear and could be a result of the treatment conditions or an artifact of the experimental design. However, the fact that urchins within the future / future treatment exhibited responses to conditions and diet similar to urchins in comparable treatments (e.g., decreased growth compared to PU, increased growth compared to PK, decreased gonad size compared to PU) suggests that the observed responses are real effects of the treatment conditions. Though these data cannot fully explain the cause of the observed effects on sea urchins, they clearly show the potential for climate change to impact urchins both directly (by effecting their physiology) and indirectly (by affecting their food).
The potential for climate change to cause phase shifts in kelp forest ecosystems by benefiting some species more than others has been noted in other studies (e.g., Connell and Russell 2010, Hepburn et al. 2011). The observed increases in M. pyrifera photosynthetic carbon uptake and growth suggest that, at least physiologically, M. pyrifera sporophytes may benefit from a changing climate, while the decreased growth and feeding rates of S. purpuratus suggest a negative response, although these may be partially ameliorated if the urchins benefit by feeding on M. pyrifera raised under these conditions. Since kelp-urchin interactions play a dominant role in structuring kelp forest communities (Mann 1977, Dayton 1985, Harrold and Reed 1985, Leinaas and Christie 1996), physiological changes that affect the strength of these interactions in the future could alter the composition of the kelp forest. For example, phase shifts from an urchin-dominated barren grounds to kelp dominated algal forests have been observed to result from a decrease in herbivory pressure, such as from the removal of herbivores by predation (Estes and Palmisano 1974) or from mass mortality (Leinaas and Christie 1996). If lowered urchin feeding rates under future conditions lead to a decrease in overall herbivory pressure, this suggests, combined with higher growth rates of M. pyrifera, a potential for greater resiliency in kelp forests following disturbances or a shift in some areas from previously urchin-dominated barren grounds to algal-dominated forests as the climate warms. However, an alternative possibility is suggested by the smaller gonad size in urchins raised under future conditions and eating kelp raised under future conditions. Here, small gonad sizes can be indicative of a low quality diet (Lemire and Himmelman 1996) or starvation conditions, and are often found in individual urchins inhabiting barren grounds (Dean et al. 1984, Harrold and Reed 1985). These barren grounds are generally believed to form when the demand for drift kelp exceeds supply, causing urchins to emerge from their cryptic habitats and actively forage. If the smaller gonad indices seen in our future conditions indicate that urchins were indeed starving climate change may lead to an increase in active foraging among urchins, exacerbating the detrimental effect they have on macroalgal growth and increasing the occurrence of barren grounds in the future.
The observed responses of M. pyrifera and S. purpuratus to multiple stressors could not have been predicted from single stressor or single species studies. Indeed, in some cases a study which examined the impact of a single stressor (e.g., elevated temperature) would have produced results opposite those seen under conditions more likely to occur in the future (i.e., elevated temperature and pCO2). Given this, the importance for climate change research moving forward in taking into account how the combined effects of biotic and abiotic stressors may impact organisms cannot be overstressed.