In 1994 Hwang and Lee described a new species of Porphyra, P. koreana Hwang & Lee (Bangiales, Rhodophyta), distinguished on the basis of habitat, colour, shape, thallus margins and arrangement of reproductive cells. In Korea, this species is known from the eastern coast, with specimens collected in an estuarine area growing in the shallow subtidal zone (Hwang and Lee 1994, 2001, Kim and Kim 2011). As part of a major revision of the order Bangiales, Sutherland et al. (2011) transferred this species to the genus Pyropia on the basis of sequence data (Pyropia koreana [M. S. Hwang& I. K. Lee] M. S. Hwang, H. G. Choi, Y. S. Oh & I. K. Lee). Recently Vergés et al. (2013) investigated foliose Bangiales in the Balearic Islands (western Mediterranean Sea), and concluded that the species Pyropia olivii (Orfandis, Neefus & T. L. Bray) J. Brodie & Neefus was conspecific with Py. koreana, and occurred on Majorca Island. Py. olivii had been reported previously from both the Mediterranean Sea (type locality Greece), as well as from New England, USA (Brodie et al. 2007).
Specimens of an epiphytic foliose member of the Bangiales have been collected over a period of 14 years from the mouth of the Avon and Heathcote rivers in Christchurch, New Zealand by Dr. Murray Parsons and coworkers. Initially regarded as a species of Porphyra, it was assigned the code “Pyropia sp. GEP” (WELT A024043) in Sutherland et al. (2011). In the two gene analysis presented in Sutherland et al. (2011) Pyropia sp. GEP and Py. koreana were resolved as sister taxa but were not considered conspecific. The data have been re-examined and on the basis of improved sequence data, it is now clear that these two entities are conspecific.
This is the first record of Py. koreana from the southern hemisphere, and is also a new record of an introduced species in New Zealand. This report summarises the features of Py. koreana in New Zealand, its morphology and currently known distribution.
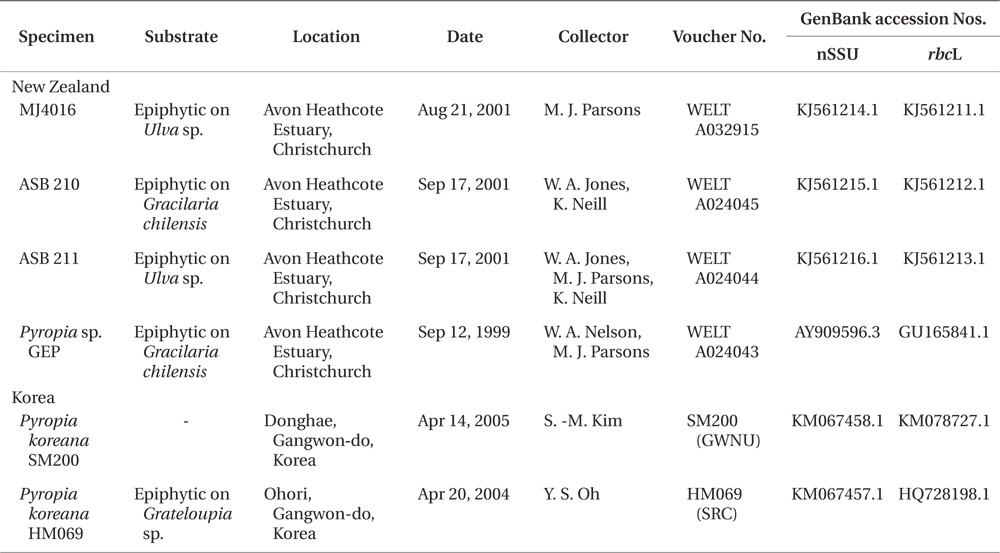
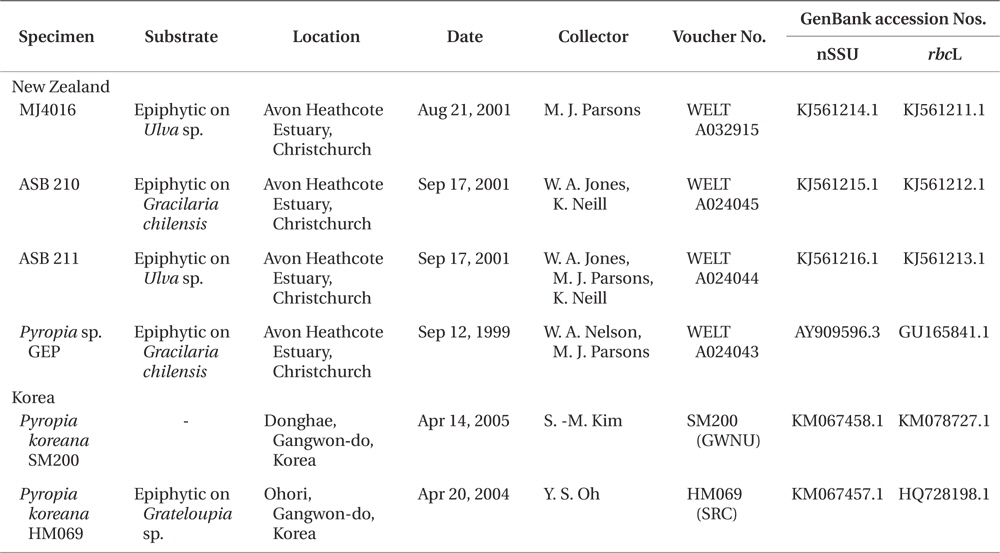
Thirty five collections have been made between 1999 and 2010 from sites close to the mouth of the Avon Heathcote estuary. Vouchers have been lodged in WELT (Herbarium of the Museum of New Zealand Te Papa Tongarewa), GWNU (Gangneung-Wonju National University Herbarium), and SRC (Seaweed Research Center, NFRDI Herbarium) (Thiers 2014).
Sequence data for the rbcL and nuclear small subunit (nSSU) genes were obtained from three additional New Zealand specimens (nSSU and rbcL genes), from the original Korean specimen, HM069 (nSSU), and from specimen SM200 (nSSU and rbcL). For the New Zealand specimens, DNA was extracted and the nSSU and rbcL loci were amplified and sequenced as in Kikuchi et al. (2010) and Jones et al. (2004). Genomic DNA was extract178ed from Korean samples using the DNeasy Plant Mini Kit (Qiagen, Hilden, Germany) and the nSSU and rbcL loci were amplified and sequenced as in Skriptsova and Choi (2009). The original sequence data from Pyropia sp. GEP (GenBank accession Nos. GU165841.1 and AY909596.1) were checked and reassessed. Collection data and GenBank accession numbers for specimens sequenced in this study are listed in Table 1. Sequences of both markers for specimens from New Zealand and Korea were aligned and compared using Geneious V6.1.6 (BioMatters, Auckland, New Zealand).
Collections were made in all months between May and November, that is, late autumn through to late spring. Small blades were found in May and June and also from October and November, with the largest blades present in July and August, reaching up to 26 cm × 10 cm or 19 cm × 14 cm. The majority of blades found were considerably smaller, ca. 0.5-1.5 cm in height. The blades were elliptical to obovate and were a rich red to purple colour in the field.
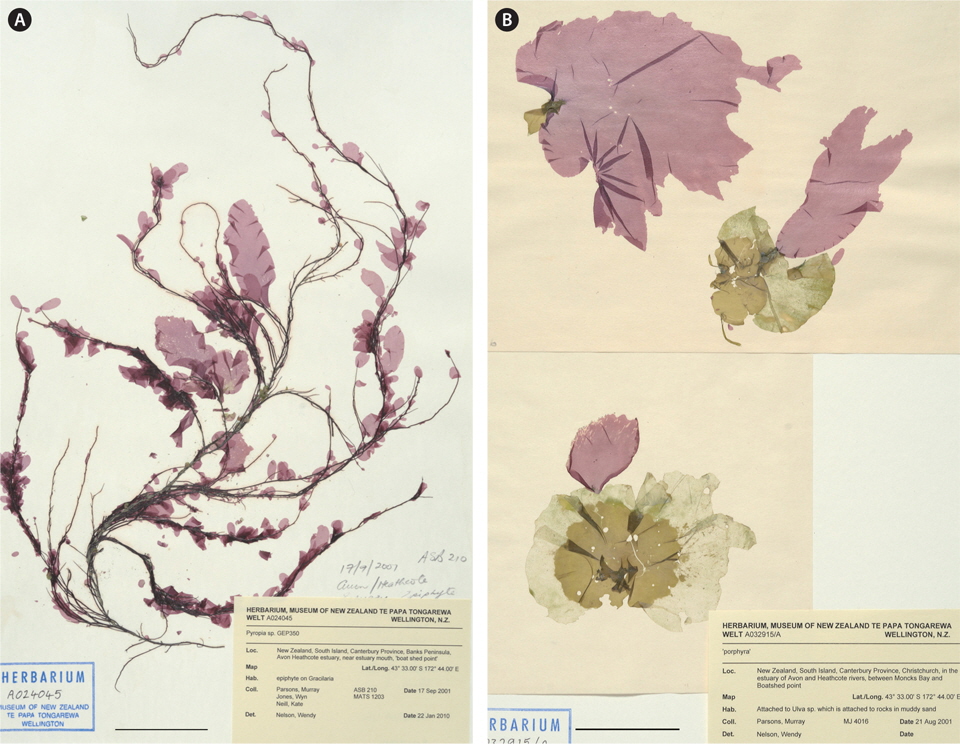
The specimens were found growing in the low intertidal to upper subtidal zones, growing epiphytically on a range of macroalgae, predominantly on Gracilaria chilensis Bird, McLachlan & Oliveira (the most commonly occurring macroalga in the estuarine area) (Fig. 1A), as well as on Ulva spp. (Fig. 1B), Gigartina atropurpurea (J. Agardh) J. Agardh, and also on Zostera capricorni Asch.
The rbcL sequences from the three new specimens from New Zealand were found to be identical to the existing rbcL sequence of Pyropia sp. GEP from New Zealand (GU165841.1), and were also identical to that of Py. koreana specimens HM069 (HQ728198.1) and SM200 (KM067458.1) from Korea.
The nSSU sequences of the three new New Zealand specimens all contained a Group I intron, as did the nSSU of Pyropia sp. GEP (AY909596.1). The four nSSU sequences from New Zealand material differed by only a single substitution within the intron, in specimen MJ4016 (GenBank accession No. KJ561214).
The new Korean nSSU sequence, from specimen SM200, also contained a Group I intron and is identical throughout both the intron and the exon to the most common New Zealand sequence. It differed, however, from the original sequence of P. koreana in GenBank (Py. koreana strain HM069, HQ728190.1). The differences all occurred in the 5 prime regions of the sequence. The original sequence, HQ728190.1, did not include a Group I intron, and there were 26 substitutions found between the first 928 nucleotides of HQ728190.1 and the sequence of SM200. BLAST analysis (Altschul et al. 1990) of the first 930 base pairs of HQ728190.1 showed that it differed by only one base pair from the nSSU sequence of a specifield men of Pyropia tenera (Kjellm.) N. Kikuchi, Miyata, M. S. Hwang & H. G. Choi (AB235851.1), also collected in Korea. The submitters have requested that this sequence (Pyropia koreana strain HM069, HQ728190.1) be removed from GenBank, and a revised nSSU sequence for HM069, KM067457.1, has been submitted (Table 1).
We conclude that the initial sequence of P. koreana was chimeric, and that the first 930 base pairs at least of HQ728190.1 were derived from Pyropia tenera or a closely related taxon. The three new New Zealand specimens, along with the first isolate, Pyropia sp. GEP, are identified as Py. koreana on the basis of homology with nSSU sequence data from Py. koreana specimen SM200, with the final 907 bp of nSSU sequence data from Py. koreana specimen HM069 (HQ728190.1), and with the rbcL sequence of specimen HM069 (HQ728198.1).
In its reported native range Py. koreana is epiphytic and found in the lower intertidal and upper subtidal zone. Thalli have been reported to be delicate and range in size up to 10 cm in length and 4-8 (12) cm in width, with a very distinctive bright red colour (Hwang and Lee 1994). Py. koreana can be distinguished from other species with similar appearance in the Northwest Pacific: Hwang and Lee (1994) compared Py. koreana with four other species native in that region. The New Zealand specimens are consistent in habit, size, and colour with the description of Py. koreana. Although the majority of thalli found to date in New Zealand fall within the size range reported from Korea, larger thalli are also present (up to 26 cm in height). Vergés et al. (2013) compared the reported dimensions and habitat of Py. koreana from the Balearic Islands, with published data from Korea and for Py. olivii (now Py. koreana) from Greece and New England, USA (based on Brodie et al. 2007). The type of Py. olivii described by Brodie et al. (2007) was growing epiphytically on Gracilaria, and they also reported this species growing on other hosts, typically in the shallow subtidal zone. Although in New Zealand there are many undescribed species of Pyropia still to be formally described that have been distinguished on the basis of molecular sequence data (e.g., Nelson et al. 2006, Sutherland et al. 2011), the epiphytic habit and the distinctive colour distinguish this species from other Pyropia in New Zealand. There are some records of epiphytic Pyropia and Porphyra within the New Zealand region, but there are no published accounts or herbarium collections of a species with this morphology.
The apparently geographically restricted distribution in New Zealand (found only in the Avon Heathcote estuary), coupled with the distinctive morphology and habit of this species, has led us to conclude that it is an introduced species in New Zealand. Although we have come to this conclusion in New Zealand, the origins and distribution of this species of Pyropia in the northern hemisphere are less clear. Brodie et al. (2007) obtained sequence data from an herbarium specimen collected in 1889 from Trieste, Italy (Adriatic), and concluded that Py. koreana (as Py. olivii) was not a “recent invader” to the Mediterranean but had been present since at least the late 19th century. Further investigations of populations in the western Atlantic, Mediterranean and north-western Pacific are required to understand the relationships between the populations within the currently known distributional range. Although described originally from Korea, this species may have a native range centred in some other part of the globe.
This record of Pyropia increases the total of red algae considered to be introduced to the New Zealand region (most recently summarised in Nelson 2012). It is not clear what impacts this species may have on receiving communities. On some of the Gracilaria host thalli there were dozens of small Pyropia blades (e.g., Fig. 1B). These may weigh the host down and cause it to dislodge and drift from the original site.
This study reinforces the value of molecular sequence data in distinguishing species, and also in the recognition of introduced species.