Hyperthermia is a promising form of cancer treatment in which the affected human tissue is exposed to >40℃ temperature for treatment [1]. Such high temperatures are intended to damage proteins and structures within the cells and to kill cancer cells. It is important that heating is localized and, of course, it must cause minimal trauma to the surrounding healthy biological tissues. To raise the temperature of the affected region, different types of energy may be used, including microwave radiation [2,3]. Typically, external or implanted microwave applicators are placed close to the body or organ to be treated. Subsequently, energy is focused on the affected tissue area to raise its temperature.
A key challenge prior to employing hyperthermia is to carry out
In this paper, we instead propose a cavity fixture to heat the culture with and without fullerene. Because of their resonant properties, cavities with metallic enclosures can achieve temperatures up to approximately 50℃ (viz. much higher than a direct waveguide illumination). Even more importantly, the temperature of the enclosed cell culture can be controlled based on the heating time and intensity. Herewith, we will adapt 2.4 GHz (Industrial Scientific and Medical [ISM] applications [8]) frequency for microwave radiation. The chosen cavity is comprised of a polystyrene cell culture dish shielded with copper, and a 2.4-GHz printed monopole antenna placed within the cavity for radiation [9]. Because of the surrounding metal shield, when the antenna is excited at 2.4 GHz, cavity resonance is achieved, giving constant rise in temperature as radiation continues. The temperature of the medium is controlled through the intensity and duration of microwave illumination. Therefore, as compared to previous works [4,10,11], the proposed set-up provides for consistent temperature increase even beyond 50℃ in a controllable manner.
This paper is organized as follows. Section II presents the design of the microwave cavity. Then, Section III gives the heating performance for different cell cultures.
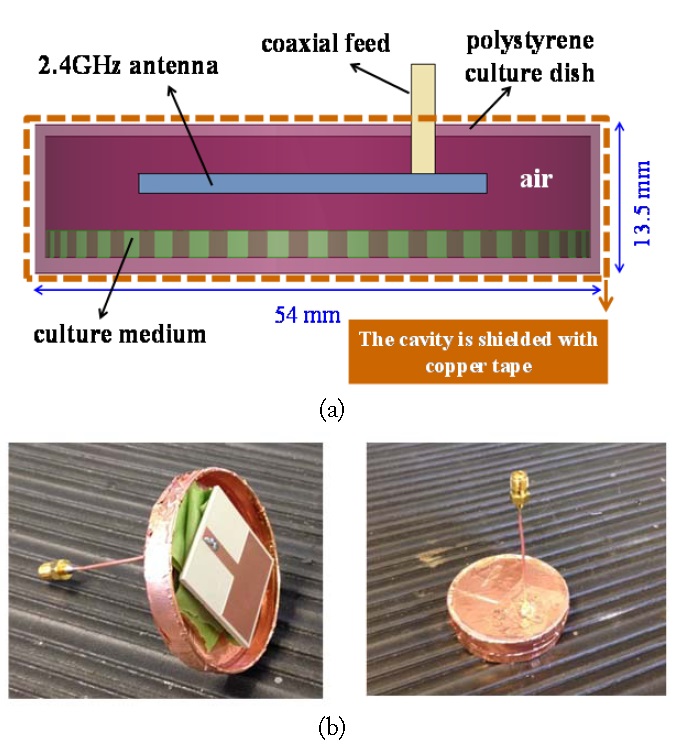
The proposed microwave cavity is shown in Fig. 1 [9]. It is comprised of a polystyrene (
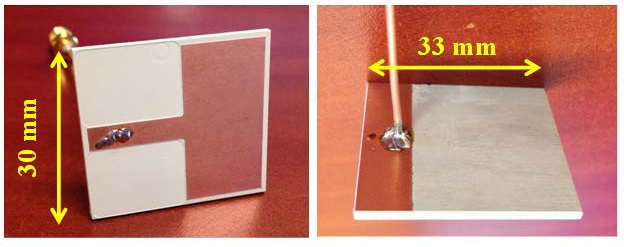
The printed monopole antenna prototype is shown in Fig. 2. It occupies 33×30×1.524 mm3 and is fabricated on Rogers TMM4 (
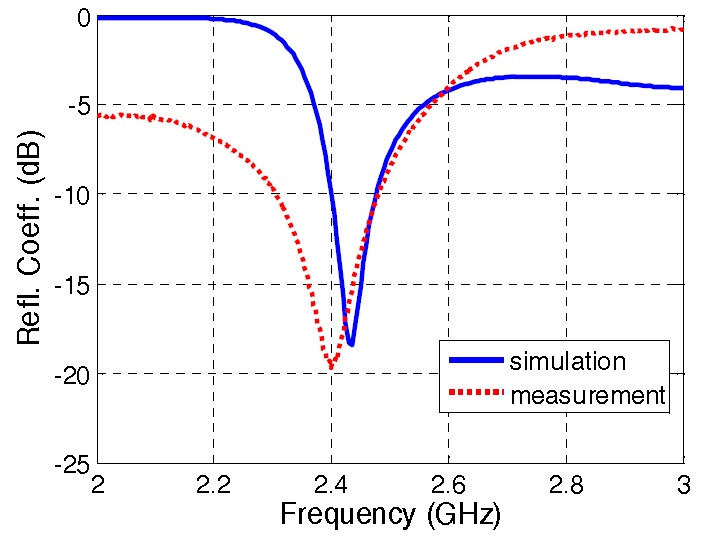
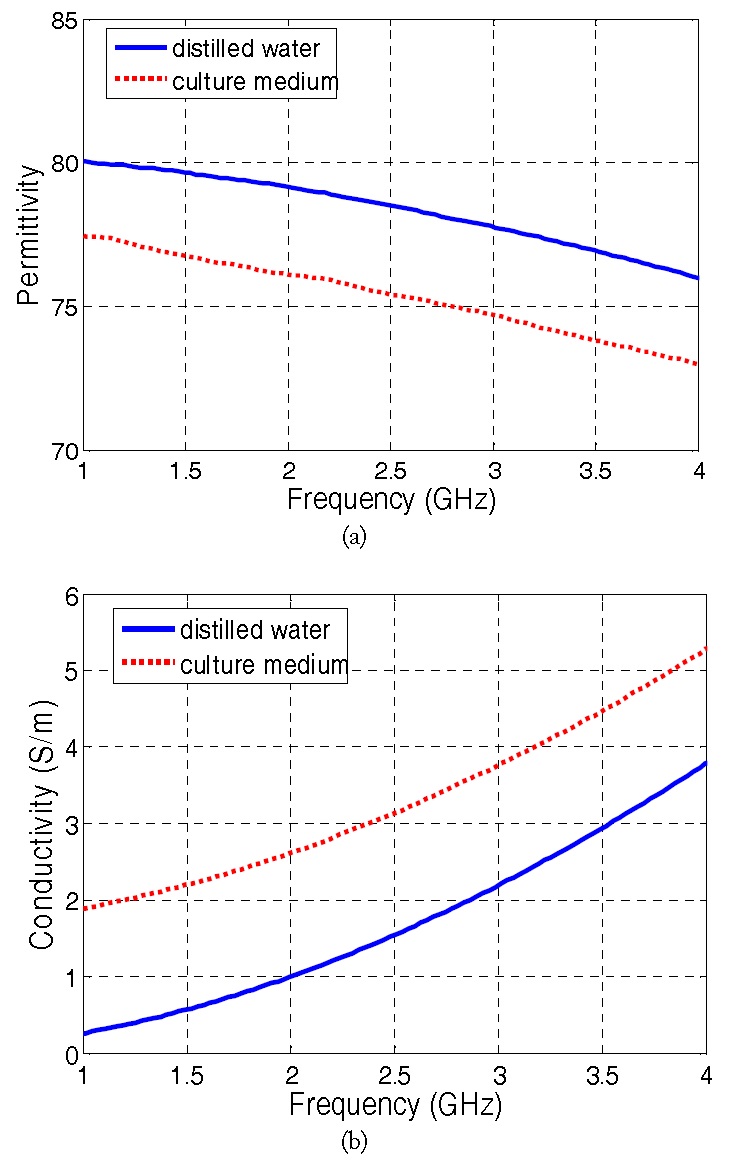
In designing the cavity and antenna, the dielectric properties (permittivity
III. THERMAL RESPONSE OF THE MICROWAVE CAVITY
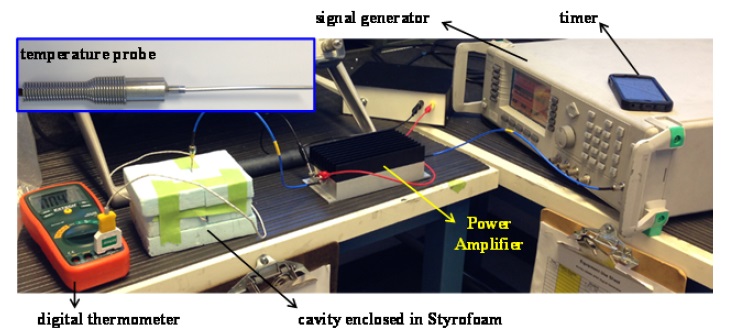
The performance of the proposed microwave cavity is evaluated by carrying out heating experiments for the F-12K culture medium (ATCC). The measurement set-up is shown in Fig. 5. A 2.4-GHz signal generator (Anritsu 68369A/NV) with a power amplifier (Mini-Circuits ZHL-42) generate 31 dBm (1.26 W) of power into the cavity. The DC power to the power amplifier is provided by a 15-V DC power supply. Also, a 2.4-GHz sleeve balun is employed between the power amplifier output and the antenna input. In doing so, a balanced operation is achieved to minimize cable radiation. The cavity contains approximately 2.75 mL of the cell culture medium (
It is necessary to include a temperature sensor, pierced through the cavity side, to sense the temperature of the enclosed medium. The temperature sensor is a custom-made ungrounded probe (diameter = 0.062ʺ, length = 2ʺ) with a type-K thermocouple at its tip (W. H. Cooke & Co. Inc., Hanover, PA, USA). Network analyzer measurements verified that the presence of this temperature sensor does not perturb the cavity’s resonance. The temperature sensor data are displayed using a digital thermometer with a resolution of 0.1℃ and an accuracy of ±0.3% (Extech TM100). We remark that for thermal insulation purposes, the microwave cavity is enclosed inside styrofoam.
Heating experiments were carried out using the measurement set-up of Fig. 5. The cavity was filled with cell culture medium (F-12K, ATCC), that was measured and then dispensed with an adjustable pipette. The initial temperature of the culture medium was 24.0℃ prior to hyperthermia. To induce hyperthermia, we turned ‘ON’ the 2.4 GHz signal generator and power amplifier to deliver 31 dBm of power at the antenna terminals. The RF generator remained ‘ON’ for 15 minutes, and the heating response was recorded as a function of time (heating cycle). The microwave source was then turned ‘OFF’ to allow the medium to cool. The exhibited cooling response was recorded for another 15 minutes (cooling cycle). We note that during the cooling cycle, the cavity was still encased with Styrofoam. All measurements were conducted at room temperature of approximately 24℃.
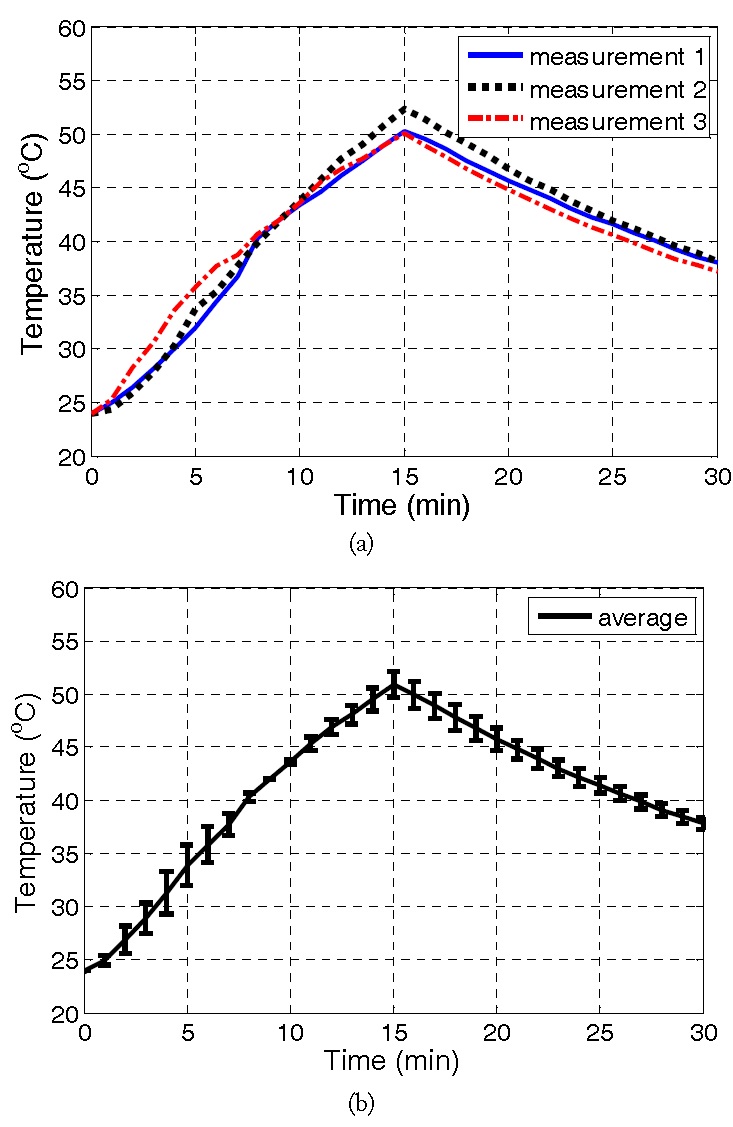
To assess the repeatability of the experiments, three sets of measurements were performed, each for different F-12K samples. The heating and cooling cycle data were then recorded and compared in Fig. 6(a). Fig. 6(b) shows the average thermal (heating/cooling) response along with error bars as a measure of the associated standard deviation (SD). We note that the SD is seen to be always less than 1.9℃, confirming the repeatability of the proposed microwave cavity set-up. On average, hyperthermia temperatures (>40℃) are reached in less than 8 minutes. Also, temperature increase from 24.0℃ to 50.9℃ is achieved within a 15 minutes heating cycle. Further, it was found that the temperature drops by about 13.1℃ (from 50.9℃ to 37.8℃) after the 15 minutes cooling cycle. Of importance is that the temperature of the enclosed cell culture medium can be controlled. To do so, the intensity (radiated power) and heating (exposure time) should be varied.
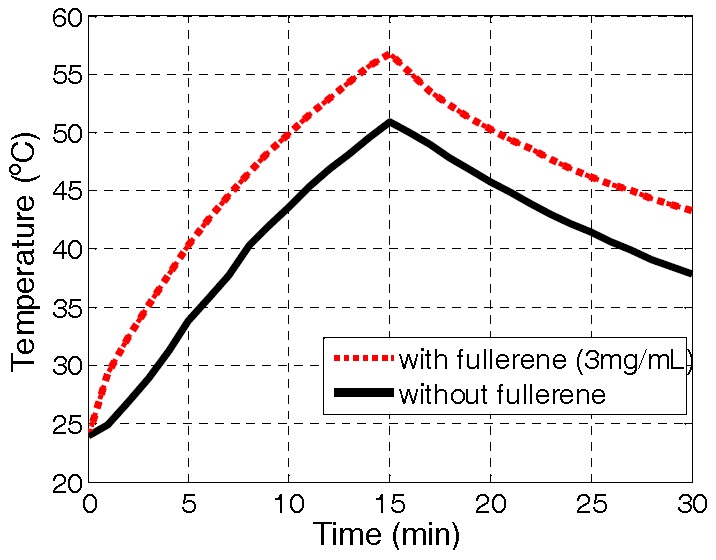
An example temperature profile of the F-12K culture medium with (3 mg/mL) and without fullerene is given in Fig. 7. Average temperature increase by 32.8℃ and 26.9℃ is achieved within the 15 minutes heating cycle, respectively. As such, fullerene agents can accelerate heating of cancer cells and improve hyperthermia treatment efficacy.
Hyperthermia is a form of cancer therapy in which the affected tissue is exposed to >40℃ temperature for treatment. Typically, the effectiveness of various hyperthermia methods can be assessed by cancer cell culturing, heating cell cultures in accordance to specifications, and recording cancer cell viability after hyperthermia. For
Heating efficacy was validated with experiments using F-12K medium (ATCC). Three sets of measurements were carried out for three separate, yet identical, samples. These measurements indicated an average temperature increase of 26.9℃ within a 15-minute heating cycle and an average temperature decrease of 13.1℃ within a follow-up 15-minute cooling cycle. Fullerene agents were shown to accelerate heating of cancer cells and improve hyperthermia treatment efficacy. Average temperature increase by 32.8℃ was achieved for F-12K medium with 3mg/mL of fullerene. As compared to previous works [4,10,11], the proposed set-up provides for consistent temperature increase beyond50℃. SD errors of less than 1.9℃ were recorded, indicating the repeatability of the set-up.
Future work will assess the effects of fullerene on cancer cell viability as a function of heating time and/or transmitted power.