Krill
The good-quality krill caught in the Antarctic Ocean need to be treated when transported to processing facilities to maintain freshness. However, krill show short-term growth at low temperatures in seawater. As activities of enzymes such as proteases, tyrosinase, and lipases are strong, deterioration of the cephalothorax and disintegration of body organization proceed within 1−2 hours when krill are piled intact on deck after fishing. Off-flavors such as fishy odor of krill due to self-digestion restrict krill use. The fish odor is a sensory quality factor for fish. Therefore, krill are immediately treated by rapid freezing after fishing in most pelagic fishing vessels to preserve their freshness (Kim, 2011).
Crustaceans such as crabs and shrimps have characteristic tastes, and their flavors are different from those of fish. Most people like their taste, and their flavor components have been of interest for processed marine products for a long time (Oh et al., 2001). Volatile compound analyses of crustaceans include flavor characteristic analysis of volatile compounds from shrimps by gas chromatography (GC) olfactometry (Lee et al., 2002) and analysis of the compounds that affect the odor of cooked shore-swimming crab flesh (Oh, 2002). Studies for use, such as enzymatic hydrolysis of crawfish processing by-products for bioflavor production (Baek, 1994), reaction technology for making natural crab-like flavors from snow crab processing by-products (Anh, 2005), and headspace volatile compound analysis of a krill reaction flavor and its application to teriyaki sauce (Kim et al., 2013), have also been conducted. However, few papers on volatile compound analysis of krill have been published so far.
Volatile compounds contributing to the odor of refrigerated krill were isolated using the headspace method, a certified method of flavor analysis to obtain basic data for quality evaluation and maintenance. Also, volatile compounds in freeze-dried krill powder were identified to compare its quality with that of fresh krill and to provide basic data for its application as an ingredient of other foods.
>
Preparation of cold-stored krill and freeze-dried krill powder
Frozen krill were obtained from Dong-Won Industries (Busan, Korea). Cold-stored krill were kept at 5°C for 0, 2, or 4 days. Freeze-dried krill were prepared by freeze-drying for 3 days. They were then crushed using a Philips HR-136 grinder (Philips, Amsterdam, Netherlands), sealed, and kept at -40°C.
>
Adsorption and desorption of headspace volatile compounds
Samples (5 g each) were put into 250-ml dark brown bottles, which were sealed, were heated in a dry oven at 50°C for 30 min, and cooled at room temperature for 1 h. The headspace volatile compounds were adsorbed for 5 min in a 90-cm stainless steel adsorption pipe (Agilent, Santa Clara, CA, USA) of compacted Tenax- TA (Supelco, Bellefonte, PA, USA) using a VPC-10 vacuum pump (Shimadzu, Kyoto, Japan) and a mass-flow controller (MFC; Shimadzu). Desorption of the stainless steel pipe was carried out using an ATD 400 automatic thermal desorber (Perkin Elmer, Waltham, MA, USA). Desorption occurred in the opposite direction to adsorption. The ATD desorption conditions were 350°C for 4 min. The desorbed gas was focused at -30°C. Then, a second desorption step (350°C for 1 min) was performed. Desorption flow was maintained at 50.2 ml/min.
>
Operating conditions for GC-MSD
The desorbed volatile compounds were automatically injected into a gas chromatograph (Shimadzu) using an auto-matic thermal desorber, separated, and identified by a Shimadzu QP-2010 plus mass-selective detector (MSD).
The AT-1 GC column (60 m × 0.32 mm × 1.0 μm; Alltech, Portland, ME, USA) was used. The temperature conditions of the oven were divided into four steps. In the first step, the temperature was kept at 35°C for 10 min; in the second step, it was increased to 120°C at a rate of 8°C/min and then maintained for 10 min. In the third step, it was increased up to 180°C at a rate of 12°C/min and maintained for 7 min. Finally, it was increased to 230°C at a rate of 15°C/min and then maintained for 10 min. The temperature of the GC interface was kept at 230°C. The carrier gas was helium of 99.9999% purity. The MSD temperature was 250°C. The mass range was set at 20 to 350 m/z. The ionization voltage was 70 eV. Carrier gas was helium of 99.9999% purity. The other analysis conditions were the same as for the GC.
The compounds for each peak in the GC-MSD total ionization chromatogram (TIC) were identified by comparing the mass spectrum with the Wiley (Hoboken, NJ, USA) and National Institute of Standards and Technology (NIST, Gaithersburg, MD, USA) mass spectra databases. Then, the Kovats retention indexes were compared. Quantitative analysis of the identified volatile compounds was carried out with the relative ratios, based on the full total peak areas for all the identified compounds.
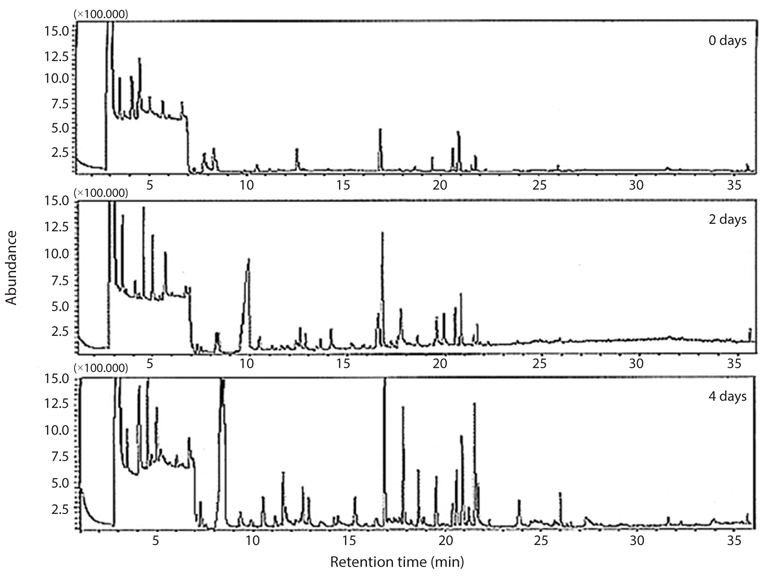
Headspace volatile compounds isolated from krill stored for 0, 2 and 4 days at 5°C after thawing using dynamic headspace analysis and their TICs are shown in Fig. 1. Total amounts of volatile compounds increased during the cold storage periods, and most volatile compounds were detected within 30 min. Interference of volatile compounds occurred at a retention time of 3−7 min, and peaks were increased or decreased. Notably, some peaks in the range of 17 to 22 min of retention time were increased.
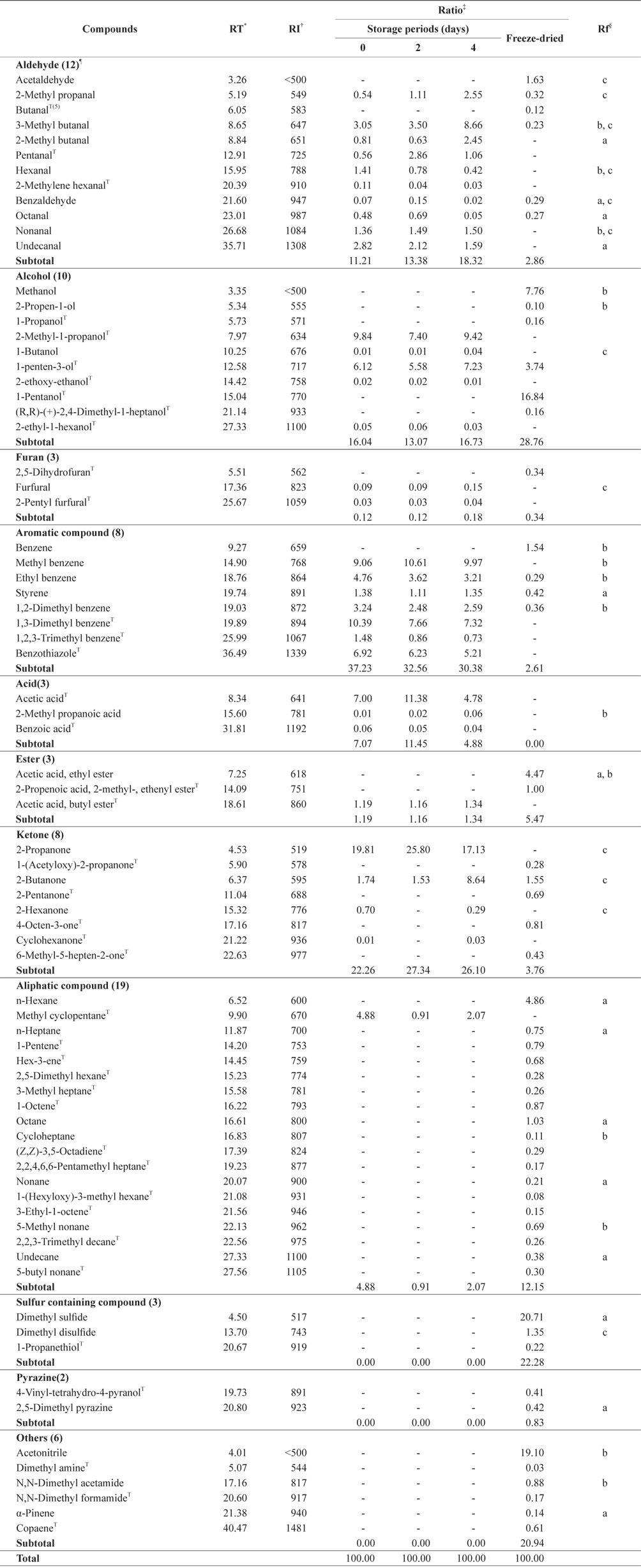
Identified headspace volatile compounds from cold-stored krill are shown in Table 1. Among a total of 33 volatile compounds, 10 aldehydes, five alcohols, four ketones, three acids, two furans, seven aromatic compounds, one ester, and one aliphatic compound were identified in the cold-stored krill.
Aldehydes have been reported to be generated by oxidation of polyunsaturated fatty acids (Lee et al., 1997). Levels of 3-methylbutanal, 2-methylbutanal, 2-methylpropanal, and nonanal increased during the storage period. Branched aldehydes such as 3-methylbutanal and 2-methylbutanal are considered to be generated by Strecker degradation of amino acids (Oh et al., 2001). These compounds were increased during the storage period in Alaska pollock and mackerel (Lee, 2011). Notably, 3-methylbutanal was identified in Korean salted and fermented anchovies (Cha, 1992). It can be predicted that 3-methylbutanal affects the odor of krill because its threshold value is very low (0.2 ppb; Lee, 2011). In fact, it was confirmed that 3-methylbutanal is directly related to off-odor according to freshness/degradation during storage (Lee, 2011). However, among C6-compounds, hexanal has a fresh smell and a good flavor (Lee, 2011), and benzaldehyde is a distinguishing flavor component of steamed crab (Cha,1992). Octanal and decanal in the stored krill disappeared during the storage period. Notably, benzaldehyde has been reported to be an important flavor component in blue crab meat, steamed crayfish, and salted fish (Cha et al., 2006). Also, benzaldehyde is known to act as a precursor to the formation of heterocyclic compounds, which are considered to be delectable flavors in food (Oh et al., 2001). However, its effect on krill odor was considered to be low because of its high threshold value and low concentration (Ahn et al., 2006).
Among alcohols, 1-penten-3-ol and 1-butanol were identified. 1-Penten-3-ol is known to have a fatty, hay, or grass flavor (Cha et al., 1999) or a rancid odor (Lee et al., 1997). 1-Butanol was detected in small amounts and is known to have a peculiar flavor, pungent and resembling a solvent (Lee, 2011). Generally, alcohols were reported to be decomposition products of secondary peroxides in fatty acids (Lee et al., 1997). Aromatic compounds were abundant among the identified volatile compounds of krill. Their levels were decreased during storage.
Ketones were identified as the second most abundant group among the headspace volatile compounds and were reported to give crustaceans a sweet or fruity flavor (Cha et al., 2006). However, in seafood, they were reported to involve a nasty smell (Lee et al., 2002). Ketones are produced by oxidation or pyrolysis of polyunsaturated fatty acids. Generally, it has been reported that ketones greatly affect the flavor of fresh fish because they have low threshold values (Cha et al., 1999). Acids are classed as wax components, and they produce a soft and mild aroma. The somewhat fishy flavor of the acids decreased during the storage period. Acetic acid was the most abundant acid. Acetic acid is reported to produce an off-flavor or a sour flavor at high temperatures (Lee et al., 2002). Aliphatic compounds, esters, and furans were identified, but only in small amounts.
Fifty-three headspace volatile compounds identified in freeze-dried krill powder are shown in Table 1. Among these compounds, there were six aldehydes, six alcohols, four aromatic compounds, two esters, five ketones, 18 aliphatic compounds, three sulfur-containing compounds, two pyrazines, and one furan.
Headspace volatile compounds in krill according to different storage periods (days) at 5°C and freeze-dried krill
Compared with the cold-stored krill, levels of aldehydes, aromatic compounds, ketones and acids were decreased in freeze-dried krill powder. Compounds with low boiling points are thought to be volatilized by the freeze-drying process, so levels of nasty compounds were rapidly decreased by heat treatment. By contrast, levels of alcohols and aliphatic compounds were rapidly increased. Alcohols have been reported to not affect the flavor of food greatly, unless they are present in large amounts, because of their high threshold values (Lee et al., 1997).
Hydrocarbon compounds such as aliphatic compounds also have high threshold values, and are thus not considered to greatly affect krill powder flavor (Oh et al., 2001). Esters are produced by esterification with alcohols and carboxylic acid. Their levels were slightly increased. Ethyl acetate was the ester with the highest content, and it was previously identified in processed fish products (Cha, 1992). Esters of low molecular weight, but not those of high molecular weight, greatly affect the sweet, fruity, and candy-like flavor of salted and fermented anchovies (Cha et al., 2006).
Unlike in cold-stored krill, sulfur-containing compounds such as dimethyl sulfide and dimethyl disulfide that are considered to be important flavor compounds in heated food were identified in freeze-dried krill (Anh, 2005). They have strong sulfur or cooked cabbage flavors in seafood products (Lee et al., 1997). Dimethyl sulfide was the most abundant sulfur-containing compound, and it is thought to be important for the flavor of sweet corn. It is produced by oxidation of methionine to methionine sulfoxide in oxidized lipids. It is reported to give onion, garlic, and cabbage flavors at very low concentrations (Lee, 2011). Dimethyl disulfide was reported to have a decayed onion flavor (Ahn et al., 2006). Sulfur-containing compounds such as dimethyl disulfide are reported to adversely affect food flavor because, at high concentrations, they mask good flavors (Ahn et al., 2006). Also, they are called aroma-active compounds because they have very low odor threshold values. Straight- chain sulfur-containing compounds are known to be produced by pyrolysis of unsaturated fatty acids and sulfur-containing amino acids (Cha et al., 2006). They have been isolated from salted and fermented anchovies (Cha, 1992).
Furans are known to be associated with burnt, sweet, and bitter flavors in processed meat and fish products (Cha, 1992). Pyrazines were reported to have a nutty, roasted, or toasted aroma in most foods (Anh et al., 2006). Their levels were slightly increased by freeze-drying. Pyrazines are generally known to be the important flavor components in crustaceans such as shrimps and crayfish (Ahn et al., 2006). Nitrogen-containing compounds such as pyrazines are considered to have distinguishing flavors because of their sensual characteristics and threshold values (Oh et al., 2001). Pyrazines are produced by Maillard or pyrolysis reactions through Strecker degradation during heating. It was reported that pyrazines levels were increased by high reaction temperatures and high concentrations (Anh, 2005). 2,5-Dimethyl pyrazine was identified in freeze-dried krill. It was previously found in potato chips, roasted peanuts, cocoa beans and baked potatos (Anh, 2005), and in red snow crab concentrate (Ahn et al., 2006). 2,5-Dimethyl pyrazine and dimethyl disulfide are known to contribute to the characteristic flavor of crab (Anh, 2005). Sulfur- or nitrogen- containing heterocyclic compounds were reported to be good flavors in crustaceans such as the red snow crab and in crayfish by-products (Lee et al., 1997).
>
Changes in volatile compounds in cold-stored and freeze-dried krill
Aldehydes, alcohols, and ketones are volatile compounds whose levels were increased by oxidation during cold storage. Notably, branched aldehydes such as 3-methylbutanal and 2-methylbutanal are volatile compounds whose levels in Alaska pollock and mackerel were increased by cold storage, and they were identified in salted and fermented anchovies. Therefore, these compounds are thought to be directly related to off-flavor according to freshness degradation during storage. By contrast, in freeze-dried krill, levels of aldehydes and ketones were dramatically decreased, while alcohol levels were increased. This is thought to have affected the off-flavor of krill because of the high threshold values for alcohols.