Chub mackerel
Fish bones may be a source of collagen, minerals, fatty acids, and other nutrients (Kim et al., 2005). It has been reported that CMB have useful components such as collagen (Nagai and Suzuki, 2000) and functional lipids (Phleger and Wambeke, 1994). So far, physicochemical characteristics of chub mackerel bones as a food resource have not been widely investigated. Studies on detail physicochemical profiles of CMB suggest the useful information to utilize them. Therefore, we investigated proximate composition, amino acid and fatty acid profiles, mineral contents, and physical properties of CMB.
The chub mackerel Scomber japonicus bones (CMB) were obtained from Gangho Fisheries Co., Ltd. (Busan, Korea). The meat and blood of bones attached to the surface of the bones were removed. The bones were washed, hot-air dried at 50°C for 12 h, and pulverized. The samples were kept frozen at -20°C until they were analyzed. All chemicals and reagents were of the highest grade available.
Moisture (oven-drying procedure), crude protein (N × 6.26), crude lipid (diethyl ether extraction), and crude ash were determined by the Association of Official Analytic Chemist (AOAC) methods (AOAC, 2005).
>
Analysis of amino acid composition
The bone powder (0.5 g) was dissolved with 15 mL of 6 N HCl in vacuum-sealed glass tubes, and hydrolyzed in a dry bath incubator (11-718-2; Fisher Scientific Co., Fair Lawn, NJ, USA) at 110°C for 24 h. After hydrolysis, the hydrolysate was filtered and concentrated to remove hydrochloric acid. The residues were dissolved with 50 mL of sodium citrate buffer (pH 2.2; Sigma-Aldrich Co., St. Louis, MO, USA), and analyzed by L-8900 amino acid auto analyzer (Hitachi, Tokyo, Japan). Values were expressed as residues/1,000 amino acid residues
>
Determination of lipid class
Total lipid was extracted according to the procedure of Folch et al. (1957) and Dyerberg (1986), and total lipid content was calculated as yield of the extracted oil. The lipid class was separated by silica gel column (2.5 × 40 cm) chromatography using silica gel 60 (70-230 mesh; Merck Co., Darmstadt, Germany). Total lipid (0.7 g) was loaded to the column, and eluted with chloroform (300 mL) and acetone (500 mL) at a flow rate of 2-3 mL/min. Finally, 300 mL of methanol were run through the column at a controlled flow rate of 1-2 mL/min. The fractions were applied by thin layer chromatography (TLC). TLC silica gel 60 plate (20 × 20 cm, 0.2 mm thick; Merck Co.) was developed with n-hexane-diethyl ether-acetic acid (80:20:1; v/v/v), and then visualized with 20% H2SO4 in order to identify lipid class. The content of each lipid class was determined by the relative percentage (w/w).
>
Analysis of fatty acid composition
Fatty acid was analyzed according to the methods of Folch et al. (1957) and AOCS (1998). Chloroform/methanol (200 mL, 2:1,v/v) was added to sample (5 g) and stirred at 60°C for 12 h. The extracted lipid (250 mg) was dissolved with 5 mL of toluene and 3 mL of BF3-methanol, and then methylated at 75°C for 30 min. The fatty acid methyl esters were extracted with 3 mL of hexane and 1 mL of 10% NaCl. The solution was filtered through a 0.45 μm membrane filter and then analyzed by gas chromatography. Gas chromatographic conditions are as follows; GC-MS QP-5050A spectrometer (Shimadzu, Kyoto, Japan) with a flame ionized detector (FID) at 250°C; Hp-INNOWax capillary column (30 × 0.32 mm i.d., 0.5 μm; Hewlett-Packard, Palo Alto, CA, USA); column oven temperature, 25-240°C (5 min hold at 100°C, 100-220°C at a rate of 5°C/min, 220-240°C at a rate of 23°C/min and 10 min hold at 240°C); carrier gas, helium (1 mL/min); split ratio, 1:20. Fatty acids were identified as compared with retention time of standard fatty acid methyl ester (Sigma-Aldrich Co.). Methyl triconoate (Sigma-Aldrich Co.) was used as internal standard. Fatty acid content was expressed as a percentage against each peak area.
>
Preparation of water-soluble calcium from chub mackerel bones
CMB was heated in a furnace (AT-E58; Isuzu Co., Tokyo, Japan) at 800°C for 6 h to give ash. The ash (1 g) was added to 25 mL of 10-50% lactic and citric acid, and then allowed to maintain at 60°C for 30 min. The mixtures were centrifuged for 10 min at 2,000 g and the supernatant was dried at 120°C.
Mineral contents of CMB were analyzed by the method of AOAC (2005) with some modifications. The bone powders (10 g) were homogenized with 20 mL of 70% nitric acid. The sample was heated for 4 h at 100 ± 5°C in a heating mantle. After cooling, the solution was made up to 100 mL using 0.2 N nitric acid. The aliquot was then assayed using an Elan 6100 inductively coupled plasma mass spectrometer (ICP-MS) (Perkin Elmer, Concord, ON, Canada). The minerals of water-soluble calcium (calcium citrate and calcium lactate) were determined by method of Tsutagawa et al. (1994).
>
Measurements of breaking strength and hardness
Breaking strength and hardness of CMB treated with different heating temperature and time were measured by a Compac-100D rheometer (Sun Scientific Co., Ltd., Tokyo, Japan). The probe was a 20 mm long disc attached to the stick, and the penetrating depth was set to 1.5 mm. Maximum weight on the load cell was set to 10 kg, and the loading rate was 60 mm/min
The proximate composition of CMB is shown in Table 1. CMB contained 40.4% moisture, 13.8% crude lipid, 15.2% crude protein, and 28.7% crude ash, respectively. Moon et al.(2009) reported that muscle of chub mackerel contained 64.8- 68.3% moisture, 5.7-10.9% lipid, 17.7-18.3% protein and 1.2-1.3% ash, respectively. CMB exhibited more lipid and ash contents than muscles of chub mackerel. From these results, chub mackerel could be investigated as a resource for fish oils or minerals extraction.
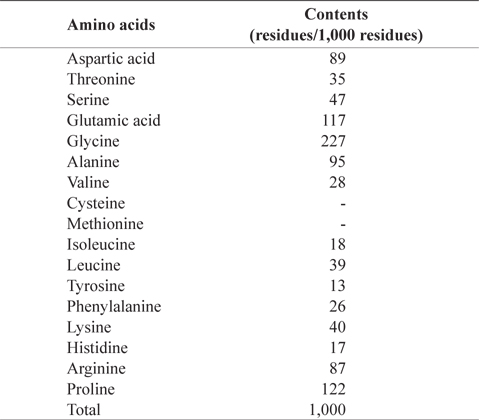
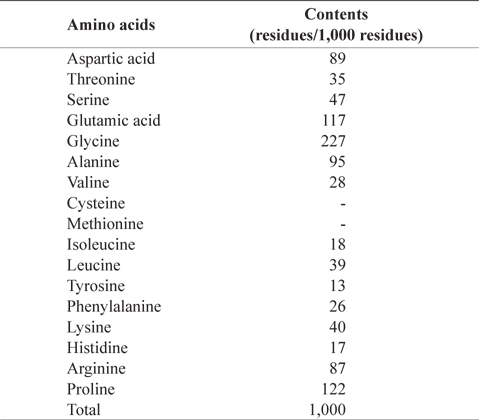
In amino acid composition of CMB (Table 2), glycine (227 residues/1,000 residues) comprised as the most abundant amino acid, followed by proline (122), glutamic acid (117), alanine (95), aspartic acid (89), and arginine (87) in order. These amino acids occupied 73% of total amino acids. CMB contains more glycine and proline than the muscles of chub mackerel because fish bone has much amount of collagen (Liaset et al., 2003). Sulfur amino acids, such as cysteine and methionine, were not detected in CMB. Oduro et al. (2011) reported amino acid profiles of mackerel muscles were rich in glutamic acid, aspartic acid, leucine and lysine, whereas contents of tryptophan, methionine and cysteine were low
[Table 1.] Proximate compositions of chub mackerel (Scomber japonicus) bones

Proximate compositions of chub mackerel (Scomber japonicus) bones
[Table 2.] Amino acid composition of chub mackerel (Scomber japonicus) bones
Amino acid composition of chub mackerel (Scomber japonicus) bones
Lipid class and fatty acid composition
The lipid class of CMB is shown in Table 3. Among lipids, the neutral lipid (94.53%) was most abundant, and contents of phospholipid and glycolipid were 3.2% and 2.3%, respectively. Moon et al. (2009) reported that neutral lipid and phospholipid contents of chub mackerel muscle are 84.3-85.0% and 15.0-15.7%, respectively. CMB had higher neutral lipid content and lower phospholipid content than the chub mackerel muscle.
[Table 3.] Lipid class profile from chub mackerel (Scomber japonicus) bones

Lipid class profile from chub mackerel (Scomber japonicus) bones
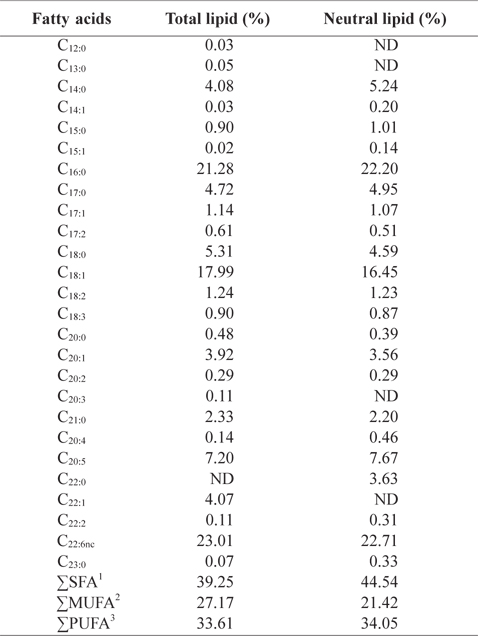
In order to better understand the lipid profiles, fatty acid compositions of total lipid and neutral lipid of CMB were analyzed (Table 4). Among fatty acids of CMB, saturated fatty ac-ids comprised 39.3% of total lipid and 44.5% of neutral lipid; monounsaturated fatty acid occupied 27.2% of total lipid and 21.4% of neutral lipid; polyunsaturated fatty acid took 33.6% of total lipid and 34.1% of neutral lipid, respectively. Major fatty acids of total lipid and neutral lipid in CMB were docosahexaenoic acid (DHA), eicosapentaenoic acid (EPA), palmitic acid, and oleic acid. Total lipid of CMB contained 21.3% palmitic acid, 18.0% oleic acid, 7.2% EPA and 23.0% DHA. Neutral lipid of CMB also showed similar contents to those of total lipid. On the other hand, in the fatty acid composition of the chub mackerel muscle, saturated fatty acid comprised 36.8-39.5% of total lipid; monounsaturated fatty acid occupied 31.8-33.4% of total lipid; polyunsaturated fatty acid took 28.7-29.6% of total lipid, respectively (Moon et al., 2009). Total lipid of mackerel muscles contained 23.0-25.2% palmitic acid, 18.3-19.0% oleic acid, 6.1-7.5% EPA and 13.7-14.3% DHA (Moon et al., 2009). Kim and Joo (1994) reported that the main constituents of fish oil were palmitic acid, oleic acid, EPA, and DHA. The CMB showed higher DHA level than the chub mackerel muscle. Lawson and Hughes (1988) reported that when DHA and EPA are ingested as triglyceride form, their absorption rates are 3-4 times higher than that of commercially available ethyl ester. This shows a potential for oil extracts from the CMB as better candidate for health supplements than ethyl ester fish oil, which has low pancreatic lipase activity.
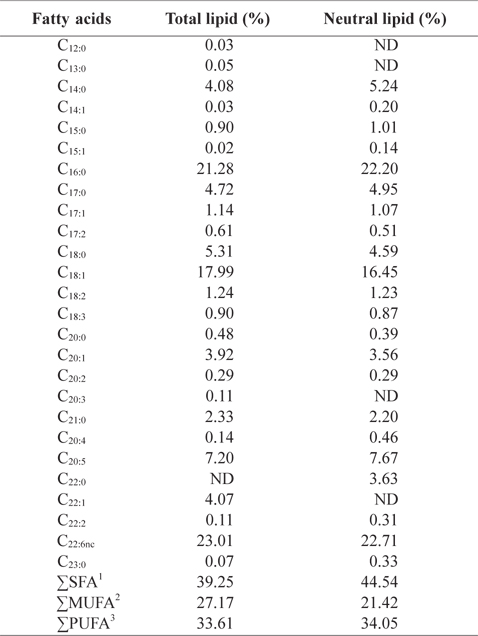
Fatty acid compositions (%) in total lipid and neutral lipid of chub mackerel Scomber japonicus bones
>
Minerals and water-soluble calcium
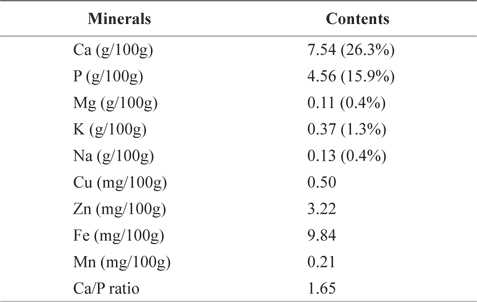
Table 5 shows mineral contents (gram per 100 g of the crude ash) of CMB. CMB contained 7.54 g/100g calcium (Ca) and 4.56 g/100g phosphorus (P). Ca and P occupied 26.3% and 15.9% of the total ash contents, respectively. Other minerals included 0.11 g/100 g magnesium (Mg), 0.37 g/100 g potassium (K), 0.13 g/100 g sodium (Na), 0.50 mg/100 g copper (Cu), 3.22 mg/100 g zinc (Zn), 9.84 mg/100 g iron (Fe), and 0.21 mg/100 g manganese (Mn). On the other hand, mineral contents of the chub mackerel muscle are ranged in 15.2-17.4 mg/100 g for Ca, 0.7-0.9 mg/100 g for P, 34.7-49.4 mg/100 g for Mn, 318.9-420.4 mg/100 g for K, 37.1-81.2 mg/100 g for Na, 0.8-1.4 mg/100 g for Zn, and 1.4-1.5 mg/100 g for Fe (Park et al., 2006). As expected, CMB exhibited higher levels of Ca than the chub mackerel muscle. Therefore, mackerel bones could be a good source for a Ca additive.
[Table 5.] Mineral contents of chub mackerel (Scomber japonicus) bones
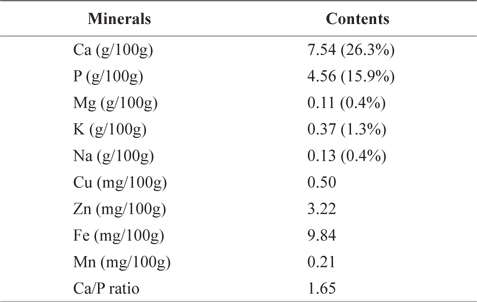
Mineral contents of chub mackerel (Scomber japonicus) bones
Lee and Kim (1998) reported that Ca and P make up human bone in 1:1.6 ratio. Ca/P ratio in mackerel bone was 1.65. Shizuki (1981) found that the Ca/P ratio in mackerel bone is similar to that in human bone and can provide benefits to human bone formation. Because the absorption rate in small intestine is limited to 60% in menopausal women, and 25-30% in average adult, Ca is considered as one of the most deficient nutrients in our body (Louie, 1996). As Ca/P ratio increased, the body absorption rate decreased (Munger et al., 1999; Barzel and Massey, 1998). Therefore, the most efficient way to consume Ca is through food (Miller et al., 2001). In our body, majority (99%) of Ca is stored in bones and teeth; the rest is stored in muscle tissue and blood.
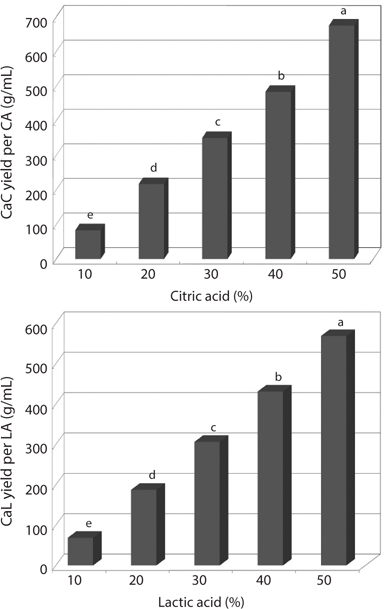
In order to prepare water-soluble calcium of CMB, the ashes from CMB were carbonized at 800°C for 6 h, and then the residues were treated with citric acid and lactic acid. Fig. 1 shows the yields of calcium citrate and calcium lactate as affected by different concentration of citric acid and lactic acid. The yields of calcium citrate and calcium lactate were increased in a concentration-dependent manner (Fig. 1). Citric acid was more effective to prepare the water-soluble calcium than lactic acid.
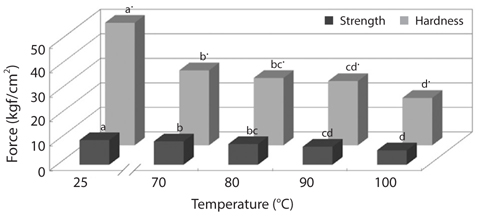
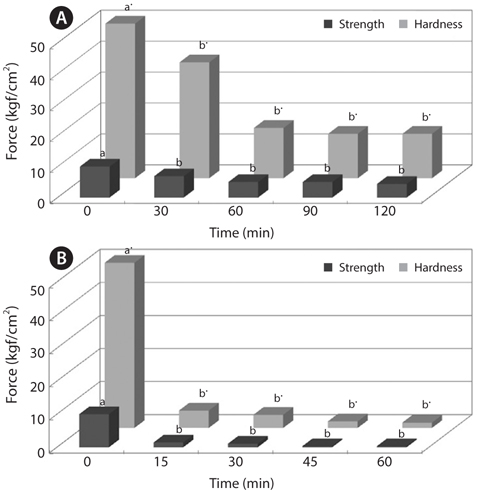
To improve convenience of the CMB intake, we investigated changes in physical properties of CMB, such as breaking strength and hardness (Figs. 2 and 3). The breaking strength and hardness of non-treated CMB were 10.01 and 50.03 kgf/ cm2, respectively, whereas the CMB heated at 100°C for 30 min showed breaking strength of 5.85 kgf/cm2 and hardness of 19.18 kgf/cm2 (Fig. 2). Generally, fish bones like mackerel are softened under the heating treatment.
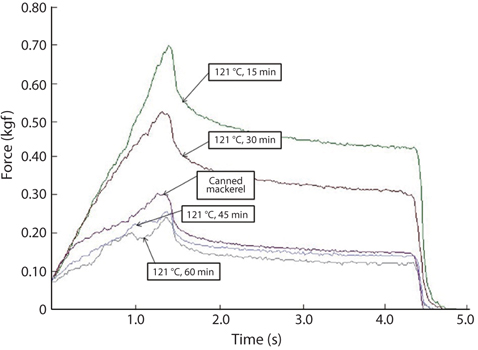
Changes in breaking strength and hardness of mackerel bones as affected by heating time at 100°C and 121°C were shown in Fig. 3. The breaking strength and hardness were sharply decreased by treatment at 100°C for 30 min and then, there was no more significant reduction by an extension of heating time after that time (Fig. 3A). In case of heating treatment at 121°C (Fig. 3B), which is a sterilization temperature of foods in an autoclave and retort, breaking strength and hardness of the bones after heating for 15 min were 5.34 kgf/ cm2 and 1.36 kgf/cm2, respectively. The bones were softened by treatment at 100°C for 30 min and at 121°C for 15 min, respectively. Moreover, CMB treated at 121°C for 45 min were softened to the similar level as the bones of commercial canned mackerel (Fig. 4).