Seaweed is a marine algae containing high levels of carbohydrates, proteins, and minerals (Indegaard and Minsaas, 1991; Fleurence and Coeur, 1993; Mabeau and Fleurence, 1993; Lahaye and Kaeffer, 1997; Rupe´rez and Saura-Calixto, 2001). Additionally, it has been used as an important food source in East Asia including China, Japan, and Korea (Xia and Abbott, 1987). A total of 28 genera of red algae have been identified, and four genera including
Koreans prefer the laver known as
Among food-pathogenic microbes, coliform bacteria are generally present in the environment including soil, surface water, and some ground water. Fecal coliforms, which are aerobic and facultative anaerobic non-spore forming, rod-shaped Gram-negative bacteria, are commonly found in the intestinal tracts of warm-blooded animals including humans. Total coliforms and fecal coliform have been used as bacterial indicators to identify contamination of human or animal intestinal tracts (Nkere et al., 2011). Total viable cell count (TVC) has been used to assess the amount of living microorganisms in a given population present in food and water, and this method has been applied by food microbiologists to test foods and production facilities for contamination (Hayes, 1992).
Dried laver can be contaminated by various sources. For example, raw laver is commonly contaminated by microbes in seawater, and cross contamination may occur through materials and by human contact during the manufacturing process of dried laver. Previous studies related to seaweed have examined market-value chain, consumption patterns, nutritional status, and residue pesticides (Lee, 2010). However, no study evaluating hazards in seaweed processing facilities has been performed. The objective of this study was to evaluate hazards in seaweed processing facilities, especially dried-laver production facilities. To achieve this objective, microbial contamination levels, including total coliforms, fecal coliforms, and TVC, as well as chemical hazards such as heavy metals, were investigated in the final processed products from dried-laver processing facilities.
To identify and evaluate food hazards,
A multiple-tube fermentation technique was used to detect total coliforms and fecal coliform in seaweed samples. This method consists of three steps including presumptive, confirmation, and completed tests, but in this study, only the presumptive and confirmation steps were used (Clesceri et al., 1998). For the presumptive test, the diluted sample was incubated in fermentation tubes of lauryl sulphate tryptose (LST) broth (Difco, Detroit, MI, USA) and incubated for 48 h at 35 ± 0.5°C. For the confirmation test, all positive fermentation tubes from the presumptive test were transferred to fermentation tubes containing brilliant green lactose bile broth (BGLB; Difco) and EC medium (Difco) using an inoculating wood stick. After transferring the positive samples, BGLB was incubated for 24–48 h at 35 ± 0.5°C, and EC was incubated in a water bath for 48 h at 44.5 ± 0.2°C. After incubation, a tube showing both growth and gas was considered positive. A most probable number (MPN) table was used to calculate the results of the total coliforms and fecal coliform tests.
The pour-plate technique was used to detect TVCs in laver samples. After homogenization as described above, samples were diluted with 10-fold serial dilutions and poured with nutrient agar. The plates were incubated for 24–48 h at 35 ± 0.5°C. After incubation, the number of colonies, expressed as colony forming units (CFU), was counted.
Food-borne bacteria including
>
Analytical condition for heavy metals
The contents of heavy metals including arsenic (As), mercury (Hg), cadmium (Cd), and lead (Pb) were only analyzed for the final dried-laver product. Samples were prepared by extracting 0.2 g of the final dried-laver product in 5 mL of methanol:water (50:50 v/v) in an ultrasonic bath for 20 minutes. The extract was evaporated until it was almost completely dry and then re-dissolved in 10 mL of deionized water. The samples were then filtered through a disposable 0.45-μm filter, after which 1 mL was removed, spiked with indium, and made up to 10 mL with 2% nitric acid (Branch et al., 1991). This solution was then analyzed using a nitrogen addition inductively coupled plasma–mass spectrometer (ICP/MS), Optima 3300XL (Perkin-Elmer, Waltham, MA, USA), at Pukyong Food Bio Center (Pukyong National University). All calibrant and sample solutions were analyzed in triplicate.
>
Bacteriological analysis of dried-laver products purchased from the market
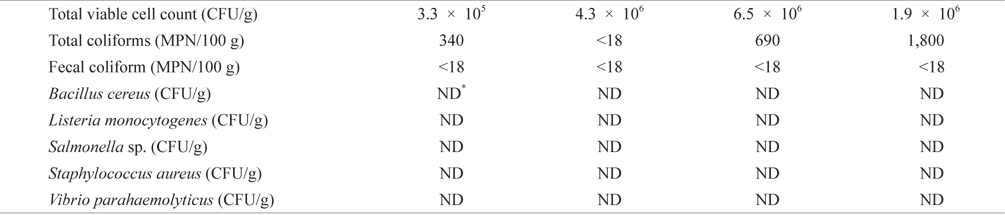
To evaluate bacteriological quality of dried-laver products distributed in the market, we purchased commercial laver products from a market located in Busan, Korea. Levels of total coliforms, fecal coliform, TVC, and major food-borne bacteria were then investigated. As shown in Table 1, TVC and total coliforms ranged from 3.3 × 105 to 4.3 × 106 CFU/g and from <18 to 1,800 MPN/100 g in samples obtained from the market, respectively. The results obtained in this study were consistent with previous studies showing that the viable cell count of dried-laver is about 106 CFU/g (An and Lee, 2000; Jo et al., 2004). Fecal coliform was detected at <18 MPN/ 100 g in all samples, indicative of an absence of fecal coliform contamination. Furthermore, food-borne bacteria were not detected in this study. The KMFDS requires TVC values of <105 CFU/g for ready-to-eat foods, although there is no TVC guideline for dried-laver products (Korea Food Code, 2013). The UK Health Protection Agency (2009) guidelines for assessing microbial safety in ready-to-eat foods on the market recommend that the level of aerobic plate count (APC) is <105 CFU/g in deli products (<5.0 log10 CFU/g); 105 to <107 CFU/g is considered borderline (5.0 to <7.0 log10 CFU/ g); and ≥107 CFU/g is unsatisfactory (≥7.0 log10 CFU/g). Additionally, food-borne bacteria including
[Table 1.] Bacteriological levels of dried-laver products purchased from market
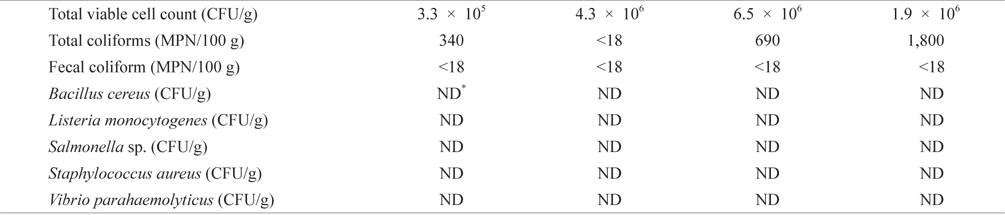
Bacteriological levels of dried-laver products purchased from market
Based on the above results, there is no serious food hazard associated with the consumption of dried-laver products, as no pathogenic bacteria or fecal coliform were detected. However, high levels of TVC and total coliform were detected in this study. Therefore, we monitored changes in the bacteriological levels during dried-laver processing to determine an effective method to reduce the high level of TVC and total coliform.
>
Bacteriological analysis of intermediates and finished products during dried-laver processing
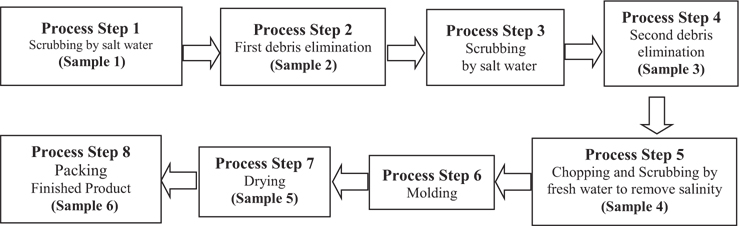
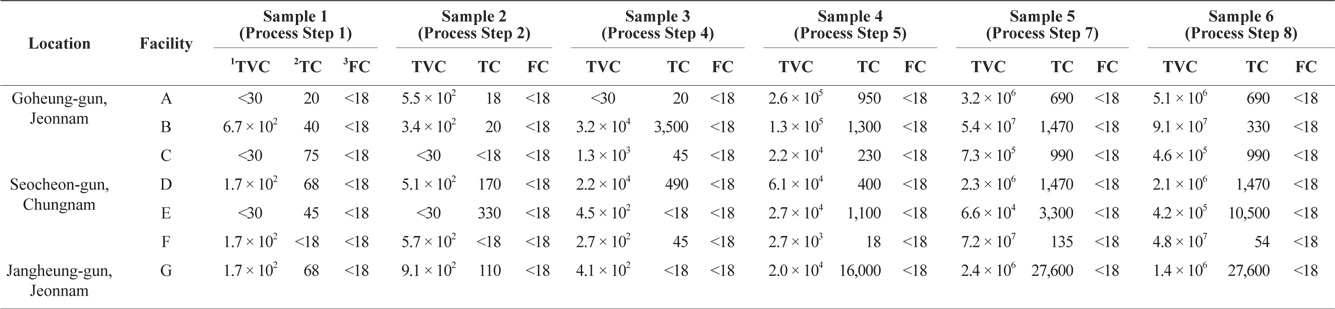
TVC analysis was performed to investigate bacteriological levels during dried-laver processing. These results can be used to identify the processing step that is most likely to cause bacteriological contamination during dried-laver processing. Samples from the seven facilities were collected at the indicated steps, as described in Fig. 1. As shown in Table 2, the TVC levels of dried-laver processing facilities were in the range <30 to 9.1 × 107 CFU in both intermediates and finished products. These results were consistent with previous studies showing that the viable cell count of dried laver exceeded 106 CFU/g (An and Lee, 2000; Jo et al., 2004). Total coliforms ranged from <18 to 27,600 MPN/100 g. The levels of TVC and total coliform gradually increased as the processing steps progressed. However, no fecal coliform was detected in the samples, as shown in Table 1.
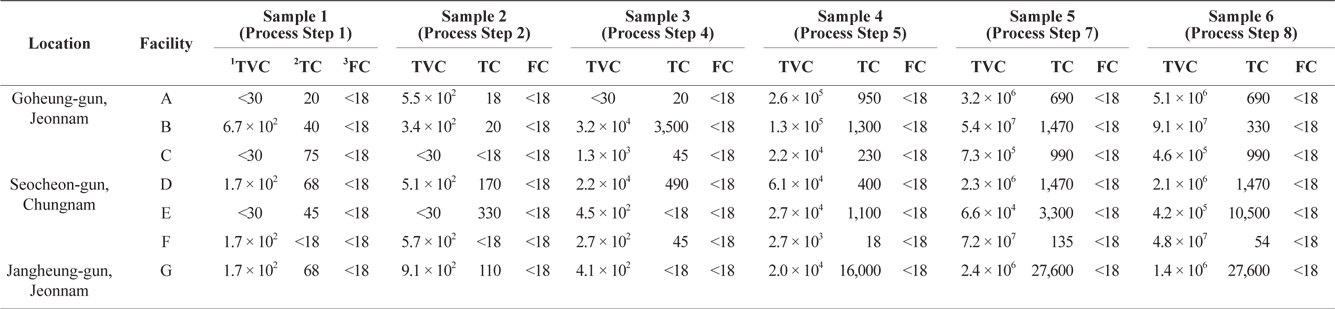
Bacteriological levels on intermediates and finished products obtained in dried-laver processing process
TVC levels significantly increased during processing step 7 (sample 5), at which time the TVC levels in most dried-laver facilities exceeded 105 CFU/g. As mentioned above, the KMFDS requires TVC values <105 CFU/g for non-heat treated foods before consumption (Korea Food Code, 2013), and the guideline of the UK Health Protection Agency (2009) for ready-to-eat foods is <105 CFU/g based on APC.
The TVC results shown in Table 2 indicate that bacterial levels commonly increased from processing step 7 to step 8. During these steps, the moisture in raw and intermediate products was removed. Generally, the dried-laver processing facilities used sponges to dehydrate laver after molding (step 6). The average replacement interval for sponges was 2–3 days. Considering the above results, the increase in bacterial levels between processing step 7 and 8 was mainly due to the fact that sponges were used for 2 or more days without replacement. In this case, the sponge would be continuously contaminated by new products. Moreover, sponge conditions are beneficial for the growth of microbes due to the high moisture level, temperature (around room temperature), and nutrient availability. To address this issue, more detailed studies on bacteriological monitoring in the sponge used for dehydration in dried-laver processing is required. We propose the following procedures to reduce the bacteriological levels during dried-laver processing. First, the sponge should be frequently replaced. Second, sanitary dried-laver processing facilities are required.
>
Heavy metal content in the final product obtained in dried-laver processing facilities
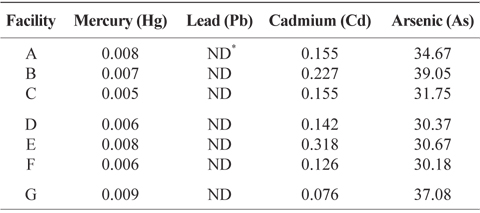
Final products obtained from seven dried-laver processing facilities were analyzed for heavy metal content consisting of As, Hg, Pb, and Cd using an ICP/MS. Table 3 indicates that the As content of final products from all dried-laver processing facilities ranged from 30.18 to 39.05 mg/kg of dried mass, Hg from 0.005 to 0.009 mg/kg, and Cd from 0.076 to 0.318 mg/kg. No Pb was detected. The Joint FAO/WHO Expert Committee on Food Additives (JECFA) recommended that the hazard quotient (HQ) determined from the ratio of exposure and safe levels of Hg, Pb, and Cd in dried-laver be <1 mg/kg dried mass (JECFA, 2010). Almela et al. (2002) reported that As contents of algae food products ranged from 0.019 to 117 /kg dried mass. Therefore, the levels of overall exposure to As, Hg, Pb, and Cd for final dried-laver products obtained from all dried-laver processing facilities were below the recommended levels, suggesting that dried-laver products are safe for public health.
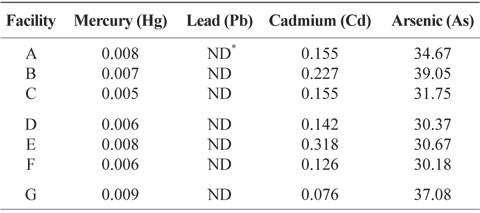
Heavy metals content (mg/kg of dry weight) of finished products obtained from dried-laver processing facilities
In conclusion, this study was performed to evaluate hazards found in dried-laver processing facilities, including bacteriological and chemical hazards. We found that the levels of TVC were higher than the guidelines established by the KMFDS and other standard guidelines for ready-to-eat foods, although though no guideline is available for dried-laver products. Based on these findings, owners of dried-laver processing facilities should use hygienic practices during processing with more frequent sponge replacement. Additionally, the authorities responsible for food safety in the Korean government should frequently evaluate the processing steps and conditions.