Marine products have substantial nutritional worth and functional properties, due to the protein fish provide 17% of the total animal protein and 6% of all protein consumed by human (Jose et al., 2007). In addition, they serve as high-quality source of vitamin and minerals, and contain high levels of polyunsaturated fatty acids (PUFAs).
Krill are small crustaceans of the order Euphausiacea, and found in all oceans. Similar to other marine animals, Antarctic krill
Despite the presence of variable functional compounds in the krill oil, however, it contains many different sorts of flavors which effect on quality of product and several studies have reported on fish sauce flavors (Peralta et al., 1996). The volatiles found in seafood consist mainly of a variety of unsaturated carbonyls and alcohols, as well as hydrocarbons, acids and esters, derived from enzymatic and non-enzymatic oxidation of PUFAs (Josephson et al., 1984; Josephson, 1991). Aromatics, phenolic compounds, sulfur-containing compounds, terpenes, pyrazines, pyridines, furans and miscellaneous compounds have also been reported in seafood (Chung et al., 2002; Grigorakis et al., 2003; Alasalvar et al., 2005) and various mechanisms for their production have been proposed (Piveteau et al., 2000; Methven et al., 2007).
Heavy metals are potentially harmful elements for most aquatic and terrestrial organisms at some concentration of uptake. Environmental factors such as pH, salinity, dissolved oxygen (DO) and temperature (Eastwood and Couture, 2002; Dural et al., 2007) as well as ecological factors such as sex, size and feed habit plays major roles in heavy metal accumulation in aquatic organisms (Turkmen et al., 2005). Fish and other marine invertebrates are considered as an appropriate bio-indicators for metals concentration in aquatic ecosystems (Domingo et al., 2007), because it could concentrate large amount of some metals in their tissues (Yılmaz et al., 2007). Heavy metal accumulation in fish may increase metals intake by human consumers, causing negative health consequences.
Microorganisms are another major concern for food safety. Both extrinsic and intrinsic parameters affect the growth of microorganisms in food products. Extrinsic parameters include environmental properties (processing and storage) external to the food product, which affect both the foods and their microorganisms. Intrinsic parameters are properties of the food product itself. For example, animal tissues can promote the growth of microorganisms under specific conditions. Refrigeration preserves food by slowing down the growth and reproduction of microorganisms and the actions of enzymes that cause food to rot. Freezing is also commonly used to preserve a wide range of foods, including those already prepared that do not require freezing in their unprepared state.
Supercritical carbon dioxide (SC-CO2) is a nonflammable, non-toxic and inert supercritical solvent, low critical temperature; it can thus be used in the food and pharmaceutical industries. In recent years, the use of supercritical fluid extraction (SFE) to remove organic compounds from various liquid and solid matrices has been explored. This technique has some advantages over more conventional separation techniques, largely due to the unique physical properties of SFs. SFE using CO2 is promising approach for the extraction and fractionation of edible oils containing labile PUFAs and lipid soluble bioactive compounds.
The objectives of this study were to extract oil from krill using SC-CO2, to provide an initial screening of volatile organic compounds in oil and residues and to compare with solvent extracted krill oil and residues. As for safety profile checking heavy metal content and microbial activity were assessed.
Antarctic krill were collected from Dongwon F&B Co. Ltd. (Seol, South Korea). Krill blocks were stored at -30℃ for less than 1 year until use. CO2 (99.99% pure) was supplied by KOSEM Co. (Gumi, Korea). All other reagents used in various analyses were analytical of HPLC grade.
Krill samples were dried in a freeze-drier (EYELA FDV-2100; Rikakikai Co. Ltd., Tokyo, Japan) for 72 h. Dried samples were crushed and sieved through a sieve (700 μm mesh size). Then crushed samples were stored at -30℃ until using for SC-CO2 and organic solvent extraction.
>
Supercritical carbon dioxide (SC-CO2) extraction
A laboratory setup pilot scale supercritical fluid extraction unit was used to extract oil from krill. This apparatus was used for extracting a large amount of oil and can be operated at pressure up to 70 MPa. Freeze-dried krill samples (600 g) were loaded into stainless steel extraction vessel, which was closed tightly with a stainless steel cap. After that extraction vessel was put into the extractor unit. CO2 was pumped at constant pressure into the extractor unit with a high pressure pump up to the desired pressure. A back pressure regulator was used to control the CO2 pressure. The extraction temperature was maintained by connecting the extractor unit to a water bath (MAT’L: SUS 304, Ilshin Autoclave Co. Ltd., Daejeon, Korea). Krill oil was extracted using SC-CO2 at different temperatures and a pressure of 40 MPa. The conditions remained constant during the 2.5 h extraction period. The extracted oil and residues were collected and stored at -30℃ until further analysis.
Ethanol and hexane were used as solvents for solvent extraction. Freeze dried and crashed samples (200g) were placed in beakers and stirred for 24 h at 40℃ at 250 rpm. After extraction, the solvent was evaporated in a rotary vacuum evaporator at 40℃. The extracted oil was collected in a vial and stored at -30℃ until further analysis.
>
Identifying of volatile compounds of krill
Krill oil was prepared using the high volume headspace vial method. Briefly, 5 mL aliquot of sample was placed in a 250 mL dark vial (Supelco Inc., Bellefonte, PA, USA). The vial was capped with a PTEE septum (QMX Laboratories Ltd., Essex, UK) and placed in an oven (50℃ for 10 min) to release volatile compounds. The sample was equilibrated for 1 h at the required temperature and the headspace gases were absorbed onto a triple-bed adsorption tube using a vacuum pump and mass flow controller (AALBORG Instrument and Controls Inc., Orangeburg, NY, USA). The triple-bed adsorbent tube consisted of a three-bed packed Tenax-TA (Supleco Inc.) with a small amount of Carbopack B (Supleco Inc.) and Carbosieve SII (Supleco Inc.) (Seo and Lee, 2010). The percentage of identified off-flavor was presented by peak area (Area%).
>
Measurement of heavy metal contents in krill
Samples were prepared as described by Khalifa et al. (2010) with minor modifications. 1 g of each sample was dissolved in 1 M of nitric acid (10 mL), boiled at 130℃ to complete the dissolution and filtered. The obtained precipitate was washed with nitric acid (1 M), transferred to a 25 mL volumetric flask and fill with de-ionized water up to the level. The heavy metals content were analyzed using PerkinElmer Elan 6100 ICPMS (PerkinElmer, Waltham, MA, USA).
>
Analysis of the microbial safety of krill residues
Colony counts were performed using spread plate method (Gilbert et al., 2000; Cappuccino and Sherman, 2002). The sample were diluted at 10-1, 10-2, 10-3, 10-4 folds in 0.9% saline water. Nutrient agar (Merck KGaA, Darmstadt, Germany) was used as growth medium for bacterial strains and sterilized at 121℃ for 15 min. The agar was poured into sterile glass Petri dishes and allowed for a moment until stiff agar forming. Nutrient agar plates were inoculated with 1 mL of diluted krill powder samples and incubated at 37℃. After 24 h of incubation, the total number of colonies was counted. Enumerated bacteria are expressed in CFU/mL (CFU= colony forming units).
The acid value (AV) was assessed according to the method described previously by Ping et al. (2008). 1 g of sample was dissolved in 100 mL of ether:ethanol (1:1, v/v) by shaking, after which phenolphthalein, as an indicator was added by drops. AV of oil was analyzed by titration with a 0.1 N KOH-ethanol solutions until the pink color persisted for at least 30 s, and it was calculated using the following equation:Acid value (AV) = 56.11 × A × F/Swhere,
The peroxide value (POV) was determined according to the American Oil Chemists’s Society (AOCS) method Cd 8-53 (AOCS, 1998) using modified amount of sample. One gram of krill oil was dissolved in 6 mL of a 3:2 acetic acid:chloroform solution. Then, 0.1 mL of saturated potassium iodine (KI) solution was added to the mixture and allowed to stand with occasional shaking for 1 min. Distilled water (6 mL) was immediately added to the solution allowed to stand. The solution was titrated with 0.1 N of sodium thiosulfate until the yellow iodine color almost disappeared. Next, 0.4 mL of a starch indicator solution was added by shaking to extract iodine from chloroform layer, and again titrated until the blue color disappeared. A blank determination was performed with the same procedure. POVs were expressed as milliequivalents of a peroxide/ 1,000 g sample:
where,
>
Identification of off-flavor compounds in krill oil and residues
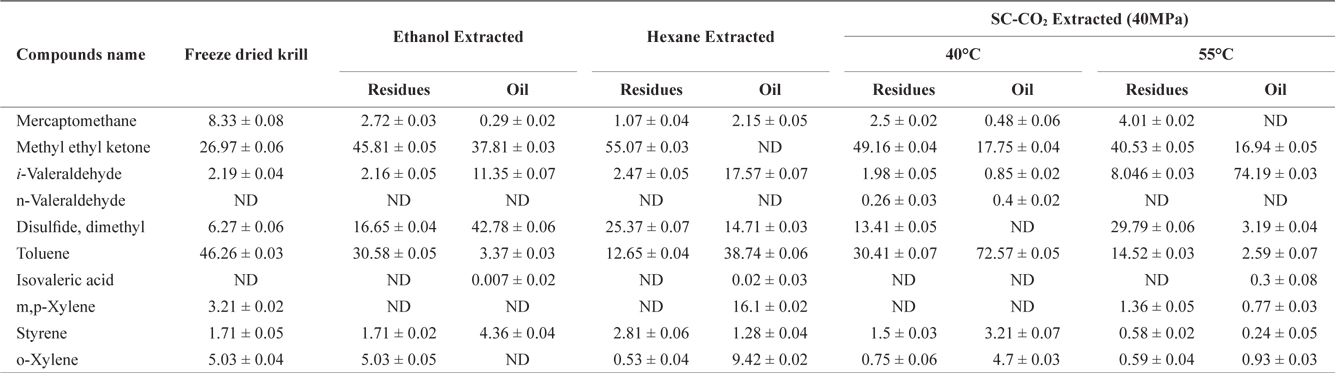
The volatile off-flavor profile was obtained for krill in the sample before and after solvent and SC-CO2 extraction (Table 1). Ten volatile compounds in total were identified. Detected compounds were classified as various chemicals, including alkenes, alcohols, aldehydes and other compounds. Methyl ethyl ketone and toluene were prominent in the freeze dried sample (before extraction) but solvent extraction decreased the concentrations of both in residues and oil. However, rest of the identified off flavor compounds were increased in both residues and oil.
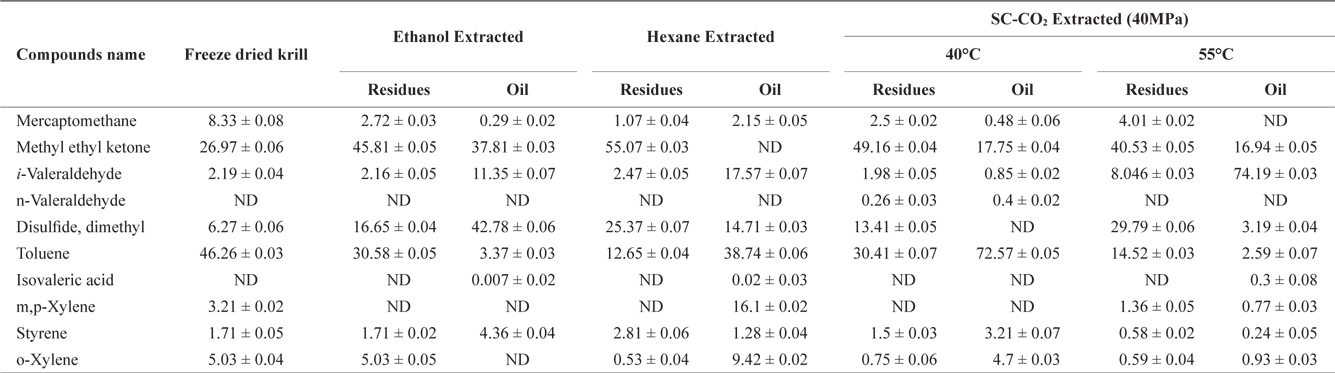
Volatile compounds and their amounta (Area%) identified in krill oil and residues after using different extraction methods
After SC-CO2 extraction, the concentration of volatile compounds decreased in oil at different temperature (40℃ and 55℃) in agreement with previous reports by Roh et al. (2006) and Lee et al. (2008). In residues the concentration of volatile compounds typically decreased, but in some cases, the concentration of volatile compounds was higher in residues than in the original sample. However, the off-flavor was more effectively removed from oil using SC-CO2 extraction compared to solvent extraction.
>
Measurement of Heavy metal contents in krill powder
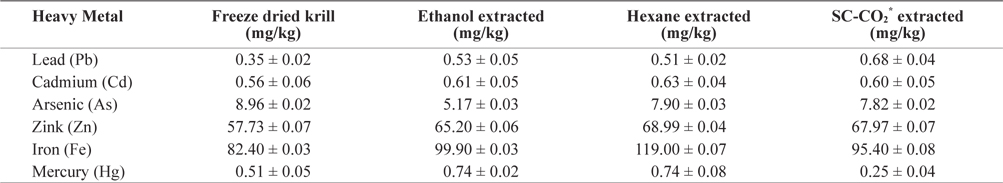
Heavy metal values of freeze-dried krill are shown in Table 2. Using different extraction methods, heavy metal concentrations in residues showed some variation. The lead (Pb) concentration before extraction was 0.345 mg/kg but after extraction, the Pb concentration increased in all extraction method and slightly exceeded the maximum level (0.4 to 0.5 mg/kg) specified by Commission of the European Communities (2002) and Australia New Zealand food Authority (2002).
[Table 2.] Heavy metals contents (mean ± SD, n = 3) analysis in different extracted krill residues
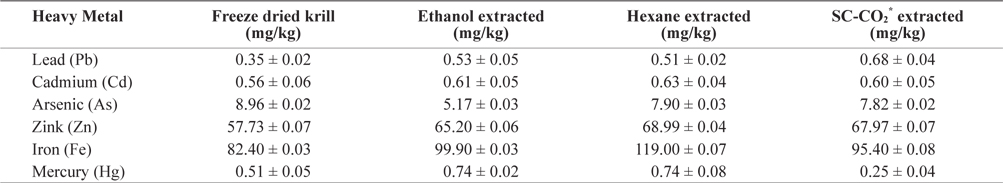
Heavy metals contents (mean ± SD, n = 3) analysis in different extracted krill residues
The mercury (Hg) concentration was 0.509 mg/kg before extraction but after solvent extraction Hg concentration was increased. In SC-CO2 extracted residue, the Hg concentration decreased (0.248 mg/kg) to concentrations bellow the restricted international standards (0.3 to 0.5 mg/kg) specified by Hong Kong government (FEHD, 1983).
The concentration of zink (Zn) also increased after extraction. The highest concentration was found in hexane extracted residues (68.99 mg/kg) and was below the maximum permissible limit (667 mg/kg) according to the Ministry of public health, Thailand (MPHT, 1986).
The concentration of cadmium (Cd) was highest (0.630 mg/kg) in hexane extracted residues and did not exceed the maximum permissible limit (1.8 mg/kg) (ICES, 1988).
In freeze dried sample the concentration of arsenic (As) was 8.96 mg/kg and lowest (5.166 mg/kg) in ethanol extracted residues. The concentration of Arsenic (As) in krill was below the permissible limit of 76 mg/kg established by the USFDA (1993).
The concentration of iron (Fe) also increased after extraction and the highest concentration was observed in hexane extracted residues. Thus, heavy metal concentration in krill was below the highest permissible limit. These levels are therefore not hazardous to human health. On the other hand, Fe and Zn were found highest amount, which are beneficial for human health. So it can be good source of Fe and Zn.
>
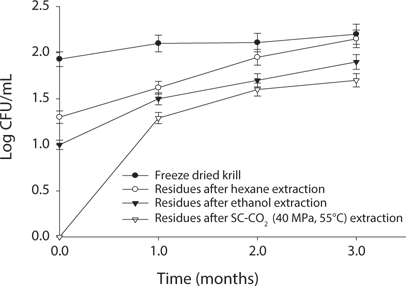
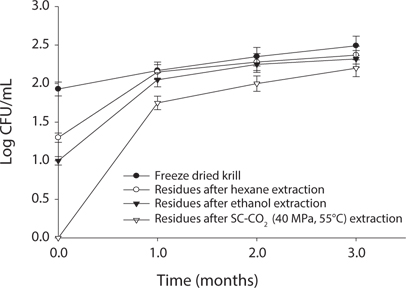
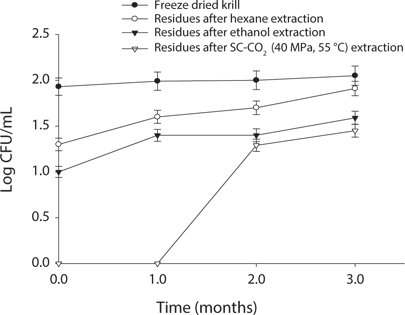
Measurement of microbial safety
Microbial safety is an important indicator to determine the quality of protein and storage period. Thus, freeze dried sample and extracted residues from different system were compared in certain days (90 days) interval at different temperature (5℃, -30℃, 20℃). Changes in total bacterial levels in oil extracted residues at different storage times are shown in Figs. 1-3. The total number of bacteria was increased in residues with storage period. It was found that SC-CO2 extracted powder showed lowest microbial growth at different temperatures during storage. High pressure CO2, various types of microbial activity can reduce the thing have been reported, and this inactivation mechanism of carbon dioxide, the fluidity and permeability with an increase in their essential areas destroyed as easily through the membrane can penetrate (Isenschimid et al., 1995). Additionally, under pressure, excessive CO2 passes through cell membranes and pH buffering capacity of cell gradients and proton motive forces fail due to lower internal pH (Hong and Pyun, 1999). However, SC-CO2 extraction method can be good method than solvent extraction method to maintain the krill product for long time storage.
>
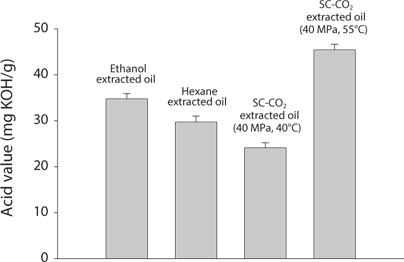
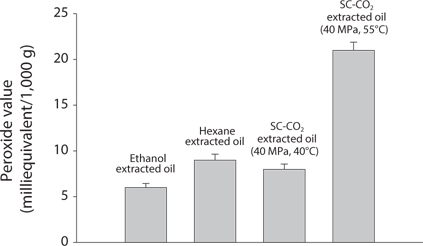
Comparison of Acid value (AV) and Peroxide value (POV) of krill oil
AV and POV were determined to measure the oil quality and the oxidation state of lipids. It is used to measure the rancidity which occurs by auto oxidation state of lipid. POV increases when the oil is oxidized and AV increases when it is hydrolyzed. It was found that with increasing temperatures, both the AV and POV increased (Figs. 4 and 5). That result proportionally associated with free fatty acid (FFA) contents (Kanai et al., 2010). In supercritical CO2 extraction, all the values were found lowest at 40℃.