Polysiphonia Greville is a large, morphologically heterogeneous group of red algae. Kim and Lee (1999) attempted to resolve some of this heterogeneity by segregating some of the species that are not considered to represent Polysiphonia sensu stricto into the new genus Neosiphonia Kim et Lee. Subsequently, it was established the Polysiphonia sensu stricto group, which has been confirmed by analyses of nuclear-encoded 18S rDNA (small subunit) sequences (Choi et al. 2001, Mamoozadeh and Freshwater 2011).
The type species of Polysiphonia is P. urceolata (Lightfoot ex Dillwyn) Greville, a synonym of P. stricta (Dillwyn) Greville. Polysiphonia sensu stricto is characterized by having ecorticate axes with 4 pericentral cells, rhizoids in open connection with pericentral cells, four-celled carpogonial branches, spermatangial branches replacing the whole trichoblasts, and tetrasporangia arranged in straight series (Maggs and Hommersand 1993, Kim et al. 2000, Choi et al. 2001). Currently, the combination of morphological and molecular analyses have confirmed the following Polysiphonia sensu stricto species from around the world: P. atlantica Kapraun et J. Norris, P. caespitosa (M. A. Pocock) Hollenberg, P. decussata Hollenberg, P. devoniensis Maggs et Hommersand, P. funebris De Notaris ex J. Agardh, P. kapraunii Stuercke et Freshwater, P. macrocarpa (C. Agardh) Sprengel, P. morrowii Harvey, P. pacifica Hollenberg, P. pernacola N. M. Adams, P. rudis J. D. Hooker et Harvey, P. scopulorum Harvey, P. senticulosa Harvey, P. stricta (Dillwyn) Greville, and P. subtilissima Montagne (Mamoozadeh and Freshwater 2011, Nam and Kang 2012, Bárbara et al. 2013, Díaz-Tapia and Bárbara 2013).
Yoon (1986) published a study on the morphology of 15 Polysiphonia sensu lato species from Korea, but only 5 of these species are currently recognized as members of the Polysiphonia sensu stricto group: P. atlantica, P. morrowii, P. scopulorum, P. senticulosa, and P. subtilissima (Lee and Kang 2002). The vegetative and reproductive morphology of most of these species has been well characterized in Korea (Kim et al. 1994, Kim and Lee 1996, Nam and Kang 2012), but none has been included in molecular analyses.
We collected unidentified samples of Polysiphonia sensu stricto from Sadongri, Ulleung Island, Korea in the spring of 2013. In this study, we characterize these samples morphologically and examine their phylogenetic relationships by analyses of rbcL sequences.
Samples were collected from Ulleung Island, Korea, during April 2013. They were preserved in 4-5% formalin / seawater for morphological study and in silica gel for molecular study. Microscope observations were made on materials stained with 1% aqueous aniline blue acidified with 0.1% diluted HCl. Photomicrographs were taken under an Olympus microscope (BX51TRF; Olympus, Tokyo, Japan) with an Olympus DP71 camera. A total of 25 individuals from 5 tufts were selected for the determination of quantitative characters and their means and standard deviations were calculated. Voucher specimens were deposited in the herbarium of Chosun University (CUK), Korea.
Genomic DNA was extracted from silica gel-dried samples using the NucleoSpin Plant II kit (Macherey-Nagel, Duren, Germany), following the manufacturer’s instructions. Polymerase chain reactions (PCR) were set up with a final volume of 20 μL using 2.5 μL of genomic DNA, 2 μL of 10 mM dNTP mix, 2 μL of 10× reaction buffer, 1 μL of 5-10 μM forward and reverse primers and 0.2 μL of TOP DNA polymerase (Bioneer, Daejeon, Korea). The rbcL locus was amplified using the primer combination F7-R753 and F645-Rrbcst (Lin et al. 2001, Bustamante et al. 2013) and purified with PCR quick-spin PCR product purification kit (iNtRON Biotechnology Inc., Seongnam, Korea). Cycle sequencing was performed with the primers F7, F645, F993, R376, R753, R1150, and RrbcStart (Freshwater and Rueness 1994, Cho et al. 2003, Bustamante et al. 2012). Sequences were determined for both forward and reverse strands using an ABI Prism 3100 Genetic Analyzer (Life Technologies, Seoul, Korea). A rbcL sequence was obtained from Polysiphonia ulleungensis sp. nov. and was deposited in EMBL / GenBank under the accession number KJ028026. This sequence and others obtained from GenBank were initially aligned with ClustalW (Thompson et al. 1994) and adjusted manually using the MEGA5 software (Tamura et al. 2011). Maximum likelihood analyses were conducted with 1,000 bootstrap replications in MEGA5 using the GTR + Γ + I model. A Bayesian inference was performed using MrBayes 3.1.2 (Huelsenbeck and Ronquist 2001, Ronquist and Huelsenbeck 2003). The Markov chain Monte Carlo runs were carried out for 2,000,000 generations each with 1 cold chain and 3 heated chains employing the GTR + Γ + I evolutionary model, sampling and printing every 1,000 generations. Summary trees were generated using a burn-in value of 200.
Diagnosis. Thalli 0.9-1.8 cm tall, saxicolous or epiphytic, and composed of prostrate and erect systems. Axes with 4 pericentral cells, ecorticate throughout, and alternate branches which are independent from trichoblasts. Trichoblasts small and scarce and their scar cells inconspicuous and scarce. Rhizoids unicellular, in open connection with and produced from the center or the proximal end of pericentral cells. Procarps bearing a fourcelled carpogonial branch. Spermatangial branches replacing trichoblast.
Holotype. CUK9483 (Fig. 1A).
Type locality. Sadongri, Ulleung-eup, Ulleung-gun (Ul- leung Island), Gyeonsangbuk-do, Korea; 37˚28′11.04″ N, 130˚53′29.38″ E; Apr 21, 2013; collected by T. O. Cho.
Isotype. CUK9583.
Etymology. The specific epithet ‘ulleungensis’ is derived from the collection locality, the island of Ulleung.
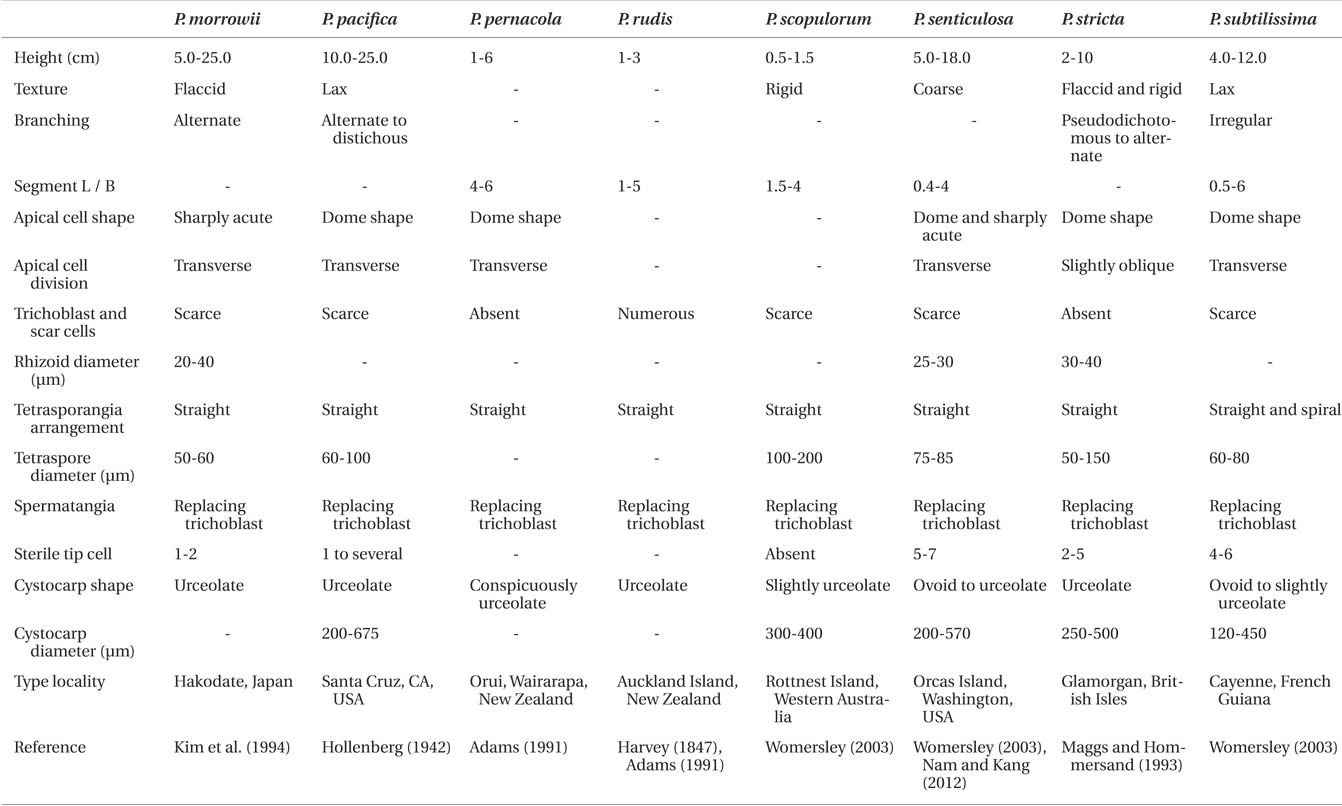
[Fig. 1.] Vegetative structures of Polysiphonia ulleungensis sp. nov. (A) Holotype specimen (CUK9483) from Sado, Dokdo-ri, Ulleung Island, Korea. (B) Habit of vegetative plant showing the extended prostrate axes and regularly branched erect axes. (C) Thallus with an alternate branching pattern. (D) Young erect axes showing short segments with alternate laterals and without trichoblasts. (E) Lower part of erect axes showing long segments without scar cells. (F) Apices showing obliquely (arrow) and transversely (arrowhead) divided apical cells. (G & H) Cross section views of erect axes (G) and prostrate axes (H). ax, axial cell; p, pericentral cell. (I & J) Rhizoids (r) showing open connection to pericentral cells (p). Scale bars represent: A, 5 mm; B, 1 mm; C, 200 μm; D, 50 μm; E & I, 100 μm; F & G, 10 μm; H & J, 20 μm.
Description. Plants are diminutive, 0.9-1.8 cm high (Fig. 1A), blackish red in color, and associated with other filamentous species. They form small tufts and are predominantly attached to rock surfaces of tide pools. Thalli are composed of prostrate and erect system (Fig. 1B). Prostate system is extensive, entangled, and decumbent with rigid texture. Segments of prostrate axes are 85.45 ± 7.56 μm long and 61.36 ± 5.45 μm in diameter, being 0.7 times broader than long (1.4 ± 0.18 in L / D). The erect system is composed of interwoven indeterminate axes. Erect axes are slender (Fig. 1E), delicate, and arise endogenously from prostrate axes at intervals of 1-4 (3.24 ± 1.39) axial cells (Fig. 1B). They are composed of 4 pericentral cells (Fig. 1G & H), ecorticate throughout and radially branched in an alternate pattern every 2-3 axial cells (Fig. 1C). Adventitious branches are absent. Young erect axes are slightly curved in the direction of the apices of prostrate axes (Fig. 1B) and have short segments (Fig. 1D). Older segments of erect axes are 70.15 ± 43.67 μm long and 35.76 ± 10.71 μm in diameter, being 0.5 times broader than long (1.98 ± 1.02 in L/D). Apical cells are prominent, 6.78 ± 1.85 μm long and 6.32 ± 1.04 μm wide, and obliquely or transversely divided (Fig. 1F). Trichoblasts are absent in vegetative thalli. Rhizoids are ventrally produced from the center or proximal end of pericentral cells of prostrate axes. They are in open connection with pericentral cells, unicellular with multilobed terminations, and 33.33 ± 12.8 μm in diameter and 180.68 ± 87.50 μm long (Fig. 1I & J).
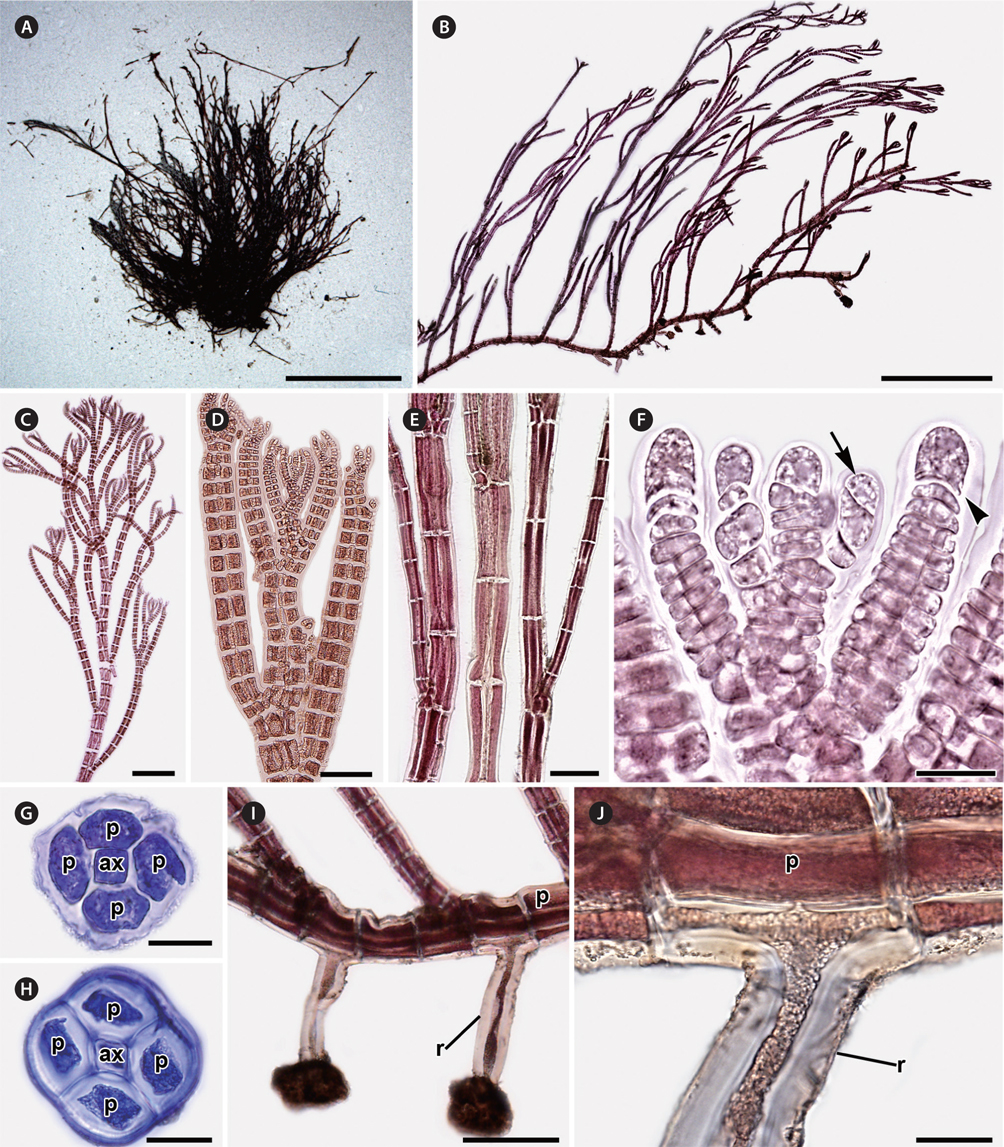
Erect axes of female gametophytes are densely branched distally and bear small, scarce trichoblasts (Fig. 2A & B). Procarps are positioned laterally and subapically on erect axes and are composed of a supporting cell bearing a four-celled carpogonial branch and a basal sterile cell (Fig. 2C). Cystocarps are elongate, slightly urceolate (Fig. 2D & E), 270.01 ± 72.73 μm high, and 200.79 ± 42.43 μm in diameter. Spermatangial branches of male gametophytes are clustered at the apices of erect axes, replace the whole trichoblast, develop one to several segments apart (Fig. 2F) and they have 2-4 sterile tip cells when mature (Fig. 2G). Tetrasporangial plants were not found.
Habitat and distribution. Plants grow from the intertidal to subtidal zones. They were found in sheltered to wave-exposed areas, and attached to rocks inside tide pools. Tufts of P. ulleungensis were entangled with filamentous algae like Antithamnion, Ceramium, and other Polysiphonia species.
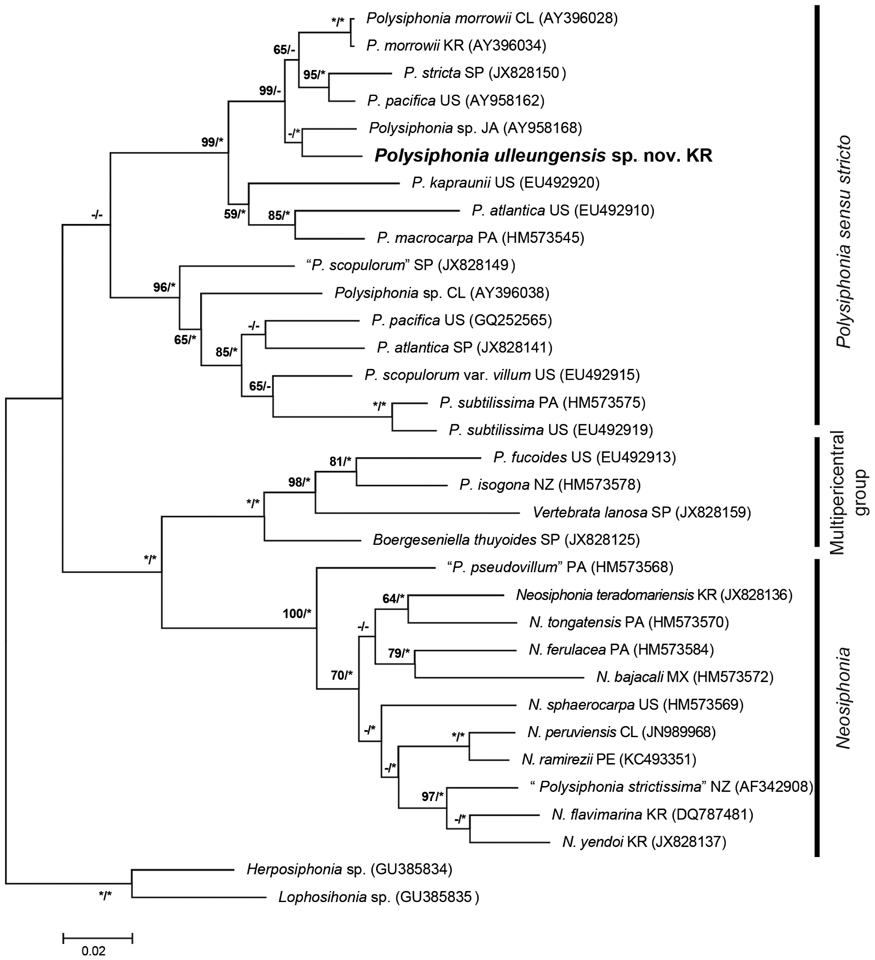
Phylogenetic analyses. A 1,245-bp portion of the 1,467-bp rbcL (84.8%) was sequenced for Polysiphonia ulleungensis sp. nov. This sequence was aligned with 31 other Polysiphonia sensu lato sequences downloaded from GenBank, and Lophosiphonia sp. (GU385835) and Herposiphonia sp. (GU385834) were included as outgroups. Phylogenetic analyses placed Polysiphonia ulleungensis sp. nov. sister to Polysiphonia sp. (EU492920) within a clade of Polysiphonia sensu stricto (Fig. 3). The sequence divergence between P. ulleungensis and Polysiphonia sp. (EU492920) was 3.2%. Polysiphonia ulleungensis diverged from eastern Atlantic and western Atlantic specimens of P. atlantica by 9.6% and 8.4%, respectively.
Polysiphonia ulleungensis sp. nov. is newly described from Sadongri, Ulleung Island, Korea, based on morphological and molecular evidence. P. ulleungensis is mainly characterized by having 4 pericentral cells, ecorticate thallus, unicellular rhizoids in open connection with pericentral cells, very scarce trichoblasts and scar cells, branches independent from trichoblasts, procarps with a four-celled carpogonial branch, and spermatangia replacing the whole trichoblast. These character states are consistent with its classification in Polysiphonia sensu stricto, and rbcL sequence analyses also support this taxonomic placement.
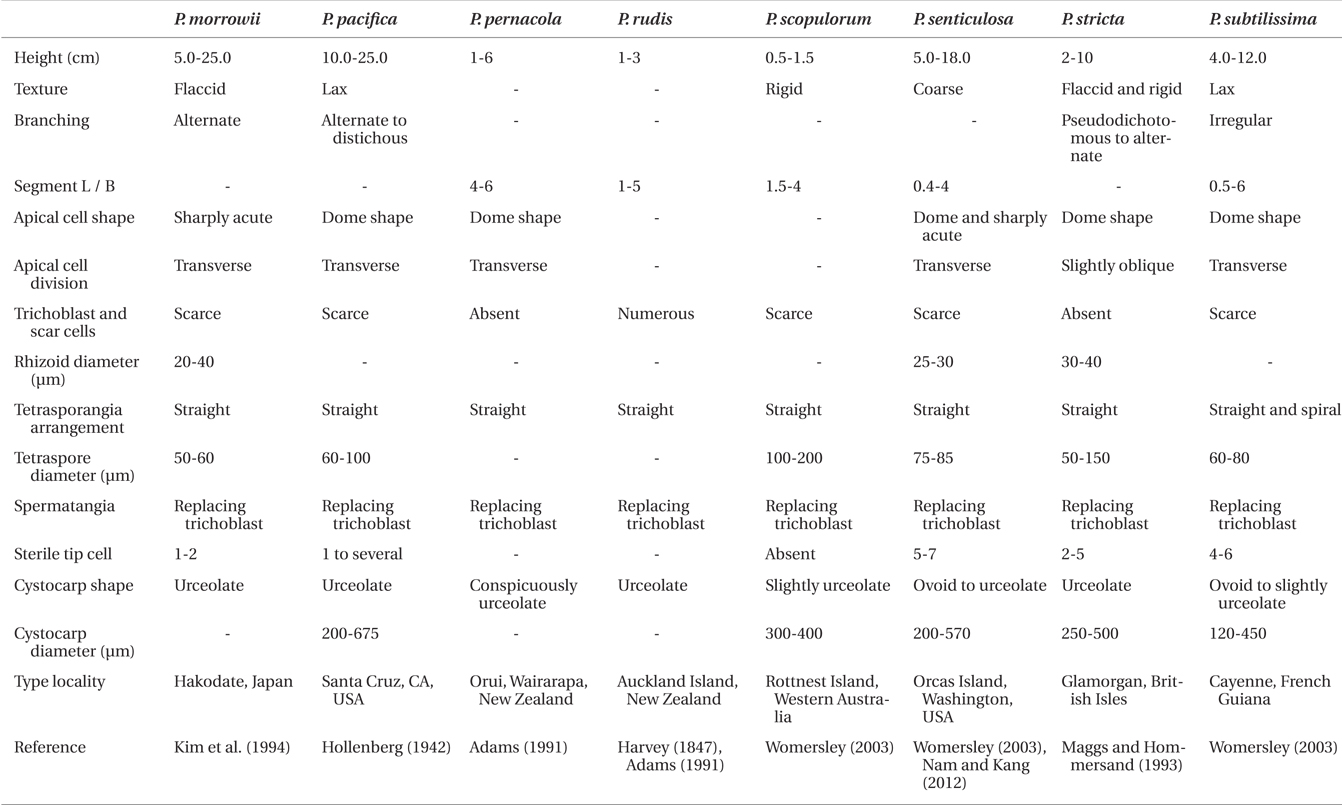
Polysiphonia ulleungensis sp. nov. is morphologically most similar to P. atlantica. P. atlantica was originally described by Harvey (1836) from Ireland as P. macrocarpa Harvey nom. Illeg. and later renamed as P. atlantica by Kapraun and Norris (1982). Maggs and Hommersand (1993) described in detail the morphology of P. atlantica from the British Isles, and European specimens were sequenced by Bárbara et al. (2013) and Díaz-Tapia and Bárbara (2013). P. atlantica has been reported from the northeastern Atlantic, western Atlantic, and Korea (Kapraun 1977, Yoon 1986, Maggs and Hommersand 1993, Kim and Lee 1996, Stuercke and Freshwater 2008, Mamoozadeh and Freshwater 2011, Nam and Kang 2012, Bárbara et al. 2013, Díaz-Tapia and Bárbara 2013). However, these records apparently involve four species, as it is explained below. Although our P. ulleungensis is similar to the European P. atlantica in having a diminutive habit and the typical features for the Polysiphonia sensu stricto members, the former is morphologically distinguished from the latter by the scarce trichoblasts and 2-4 sterile tip cells in the spermatangia (Table 1).
P. atlantica was initially reported and described from Korea by Yoon (1986), and additional detailed morphological descriptions were provided by Kim and Lee (1996) and Nam and Kang (2012). Kim and Lee (1996) reported the plants collected from Daesambudo as “P. atlantica” on the basis of field and laboratory cultured materials. However, these plants are distinguished from P. atlantica described from Europe by paired branches being borne on one side of the axis and then a pair on the other side. Polysiphonia atlantica sensu Kim and Lee (1996) has not been included in molecular analyses, and it may represent a different species of Polysiphonia sensu stricto.
Polysiphonia atlantica sensu Nam and Kang (2012) was collected from the eastern and southern coasts of Korea. It is distinguished from European P. atlantica by the scarce trichoblasts and 2-4 sterile tip cells in the spermatangia. It also differs from P. atlantica sensu Kim and Lee (1996) in having an alternate branching pattern. P. atlantica sensu Nam and Kang (2012) resembles P. ulleungensis sp. nov. based on the morphology of vegetative, male, and female thalli, although it has been reported with a dichotomous branching pattern that seems to be alternate in the tetrasporophyte (see Fig. 18H in Nam and Kang 2012). We recognize the P. atlantica from Korea reported by Nam and Kang (2012) as P. ulleungensis sp. nov.
The following seven Polysiphonia sensu stricto species are also similar to our new species: P. funebris, P. morrowii, P. pacifica, P. pernacola, P. scopulorum, P. senticulosa, and P. subtilissima by having the combined features of scarce trichoblasts and scar cells and spermatangial branches replacing trichoblasts (Table 1). However, P. funebris differs from P. ulleungensis sp. nov. by the scattered disposition of the ovoid cystocarps throughout the female gametophyte and an alternate-distichous branching pattern (Afonso-Carrillo and Rojas-Gonzalez 2004). Polysiphonia morrowii is distinguished by the sharply pointed vegetative tips and 5 to 8 sterile tip cells in spermatangial branches (Kim et al. 1994, Geoffroy et al. 2012). P. pacifica is distinguished by the lax texture and distichous branches (Hollenberg 1942). P. pernacola is distinguished by the absence of trichoblasts and conspicuous urceolate cystocarps (Adams 1991). P. scopulorum differs by the rigid texture of filaments, presence of adventitious erect branches and cicatrigenous branching, absence of sterile tip cells in spermatangial branches, and slightly spiral tetrasporangia arrangement (Womersley 1979, see Fig. 18I in Nam and Kang 2012). P. senticulosa differs by the laterals hooked in a proximal direction and 5 to 7 sterile tip cells (Nam and Kang 2012). P. subtilissima is distinguished by its lax texture, irregular branching, and both straight and spiral arrangement of tetrasporangia (Womersley 2003).
Molecular-assisted identification with plastid-encoded rbcL has proven useful for discriminating species of Polysiphonia sensu lato (Mamoozadeh and Freshwater 2011). Our molecular phylogenetic analyses using rbcL sequences reveal that P. ulleungensis sp. nov. is firmly embedded within Polysiphonia sensu stricto (Fig. 3). P. ulleungensis is situated in a well-supported clade with the generitype, P. stricta, and other species that have in common the rhizoids in open connection to pericentral cells, 4 pericentral cells, and branches radially emerged. P. ulleungensis shows sufficient sequence divergence from morphologically similar P. atlantica from Europe, “P. atlantica” from the USA, and other Polysiphonia species to warrant recognition as a new species. P. ulleungensis sp. nov. has been collected Ulleung Island (~135 km off the eastern coast of the Korean Peninsula) and it was also reported from the western and eastern coasts of Korea as P. atlantica sensu Nam and Kang (2012).