Scanning electron microscopy (SEM) has facilitated reliable differentiation among species using fine cell structures (Sala et al. 2006). The first detailed account of some species (
Nuclear-encoded small subunit (SSU) rDNA sequences are now available for representatives of most major diatom lineages (Medlin et al. 1993, 1995, 1996, Kooistra and Medlin 1996, Beszteri et al. 2001, Ki et al. 2009, Jung et al. 2010). Some previous studies have addressed diatom evolution and systematics using SSU rDNA (Damsté et al. 2004, Medlin and Kaczmarska 2004, Bruder and Medlin 2008), in which they analyzed some species of
In this paper, we provide light and scanning electron microscope data for eight diatom species of
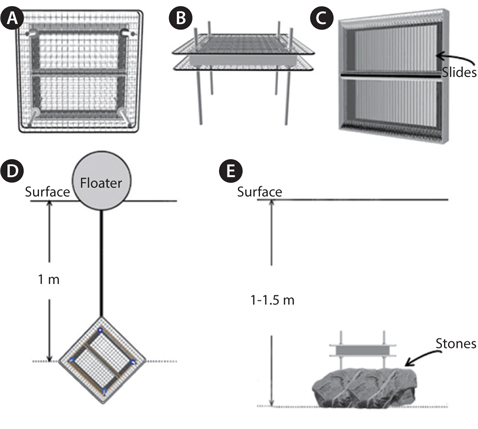
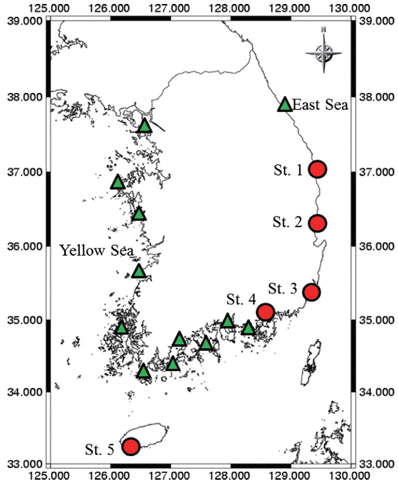
Samples were collected with an algae-harvesting tool (Fig. 1) or by brushing algae from stones at 17 coastal Korean sites (Fig. 2). Species of
>
Fixation and frustule-cleaning
Natural and cultured samples were fixed in Lugol’s solution (Throndsen 1978) with a final content of c. 2%, for at least eight hours at 4℃. Before frustule-cleaning, the cells were rinsed with distilled water. Organic compounds were removed from fixed cells by concentrating samples to discard the supernatant, adding an equal amount of HNO3 and three times the sample amount of H2SO4, and finally boiling for three minutes. Next, the samples were rinsed with distilled water to completely remove the acid (Ki et al. 2009).
Material from cleaned samples was mounted in Naphrax for morphometric analysis; the slides are preserved in the Laboratory for Water Environmental Ecology and Restoration at Hanyang University, with accession numbers AM001-AM008. Light microscope observations were made on slides from exponentially-growing cultures and / or natural samples using an Axioplan microscope (Carl Zeiss) equipped with Nomarski differential interference contrast optics. Light micrographs were taken using a cooled CCD camera (Sensys Photometrics, München, Germany) and analyzed with Image-pro Plus 6.0 software (Media Cybernetics, Silver Springs, MD, USA). Diatom cells in the exponential growth stage were examined for cell length, chloroplast shape and nucleus position. Average cell length and width were calculated from measurement of more than 30 cells. We used the striae-counting method outlined in Schoeman and Archibald (1976).
>
Scanning electron microscopy (SEM)
To determine the actual shape and fine structure of the diatom frustules, both the fixed specimens and the specimens with organic compounds removed were observed in the SEM. The specimens were dehydrated in a graded ethanol series (30, 50, 70, 90, and 100%; each stage for 30 min). Next, 50-µL of dehydrated specimens were mounted on a 0.1% w/v (in water) poly-L-lysine solution (Sigma, St. Louis, MO, USA)-treated glass cover-slip (25 µL of poly-L-lysine solution on an 18-mm-diameter glass coverslip, uniformly coated and dried at room temperature). Or dehydrated specimens were directly mounted onto a 0.2-µm GTTP Millipore Filter Membrane (Millipore Filter Corporation, Cork, Ireland). Both treated glass and filter membranes were glued onto SEM stubs before specimen mounting. The mounted specimens were dried at room temperature for 12 h. Finally, specimens were coated with gold for 200 s with a 25-mA current (BAL-TEC SCD 005 Super Coater; BAL-TEC, Liechtenstein, Germany) and examined with the SEM (Hitachi S-2380n; Hitachi, Tokyo, Japan and JSM-6300; Jeol, Tokyo, Japan).
>
DNA extraction, polymerase chain reaction (PCR) amplification, and sequencing
Samples of clonal cultures (3 mL) in the mid-logarithmic growth phase were harvested by centrifugation at 8,000 ×g for 5 min. The concentrated cells were transferred to 1.5-mL Eppendorf tubes with 100 µL of TE buffer (10 mM Tris-HCl, pH 8.0, and 1 mM ethylenediaminetetraacetic acid) and stored at -20℃ until DNA extraction (Ki and Han 2007). Genomic DNA was isolated from the stored cells using the DNeasy Plant Mini Kit (Qiagen, Valencia, CA, USA).
For getting the SSU rDNA sequences, primers shown in Table 1 were employed in the PCR amplification. PCR reactions were performed with 50-µL reaction mixtures containing 33.5 µL sterile distilled water, 5 µL 10×
[Table 1.] Primers used for amplifications and sequencing of the nuclear SSU rDNA in this study
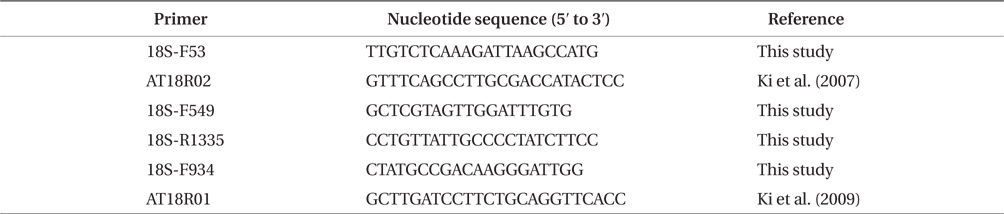
Primers used for amplifications and sequencing of the nuclear SSU rDNA in this study
Full multiple alignment of our nuclear SSU rDNA sequences with NCBI (Table 2) sequences were performed with the Clustal W1.8 (Thompson et al. 1994) portion of the Bioedit program v7.0.9.0 (North Carolina State University). The aligned nuclear SSU rDNA sequences were trimmed to the same length at each end by Bioedit. In addition, all the SSU rDNA sequences of eight species sequenced here together with other
[Table 2.] Strains in this study and GenBank accession numbers for DNA sequences
Strains in this study and GenBank accession numbers for DNA sequences
The alignment matrix was analyzed using the maximum likelihood (ML) method. To determine the optimal model of nucleotide substitution, hierarchical likelihood ratio tests (hLRTs) and calculated Akaike information criterion (AIC) values (Posada and Buckley 2004) in MrModelTest 2.3 (Nylander 2004), assisted by PAUP*4.0b10 (Swofford 2003), were used. The best-fit model (GTR + I + G) was selected from the 24 tested models for the PhyML 3.0 (Guindon et al. 2010) settings. Bootstrap values (branch support) were obtained with 1,000 replicates. Bootstrap values greater than 50 are indicated at each branch node.
For the Bayesian inference (BI) analysis, the optimal model of nucleotide substitution was determined as the method in ML analysis. The best-fit model (GTR + I + G) was selected from 24 tested models for the MrBayes 3.2.1 (Ronquist et al. 2012) settings. The Markov Chain Monte Carlo (MCMC) process was set at two chains, and 1,000,000 generations were conducted. The sampling frequency was arranged as occurring with every 10 generations. After analysis, making sure the standard deviation of frequencies occurred below 0.01, the first 25% trees were deleted as burn-in, and a consensus tree was constructed. Bayesian posterior probabilities (BI) greater than 0.50 were indicated at each branch node.
Maximum-parsimony (MP), with branch and bound searches, was done using PAUP*4.0b10 (Swofford 2003) with the hLRTs setting. Bootstrap values (branch support) were obtained with 1,000 replicates. Bootstrap values greater than 50 were indicated at each branch node.
>
Comparisons of rDNA sequences
The nuclear SSU rDNA sequences from eight
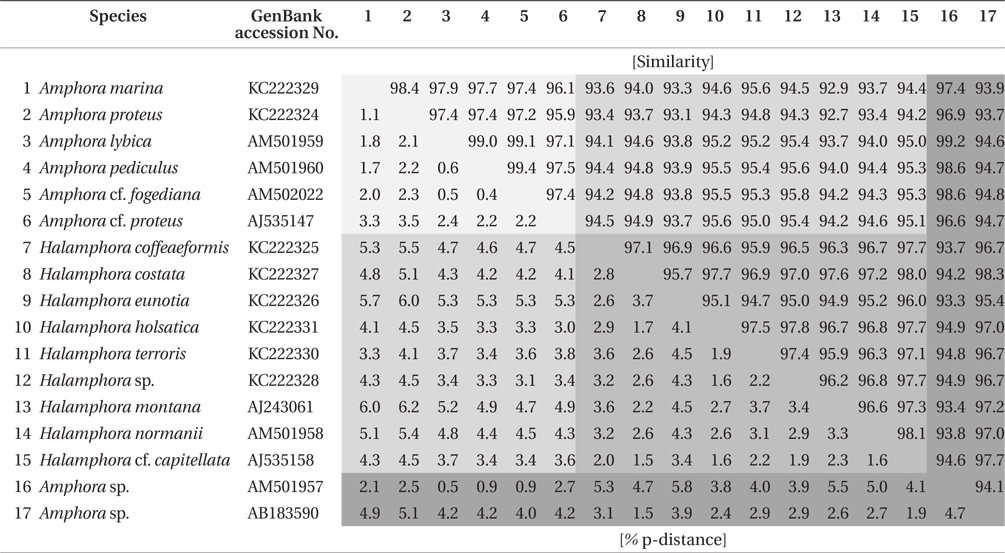
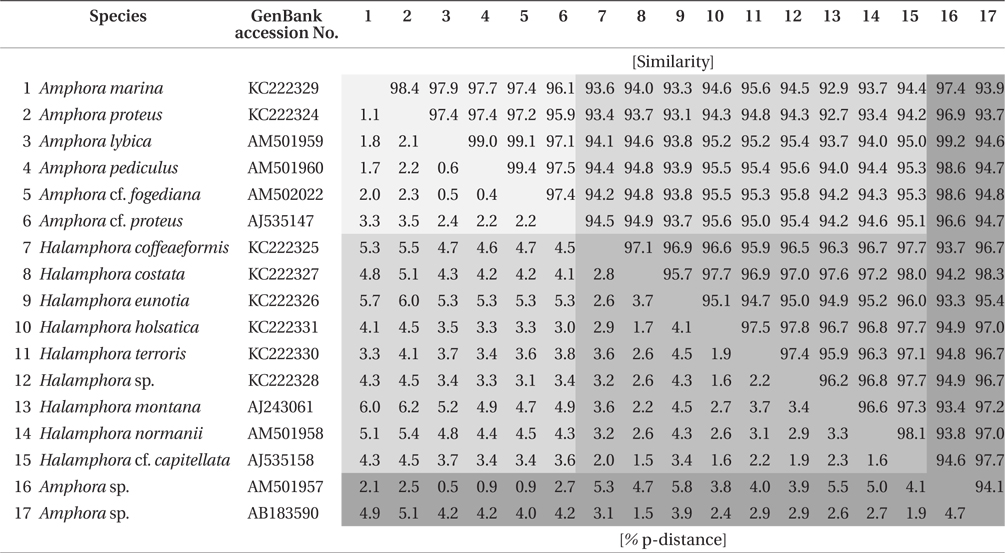
Similarity score and genetic distance of 17 pairs aligned Amphora sensu lato nuclear SSU rDNA sequence (1,667 bp)
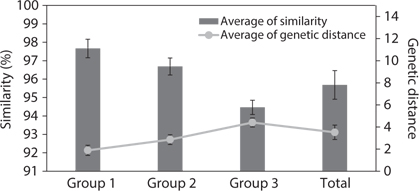
The results of ANOVA tests of similarity and genetic distance are displayed in Fig. 3. The three groups were independent and significantly different (p < 0.001). Group 1 is the similarity between species in
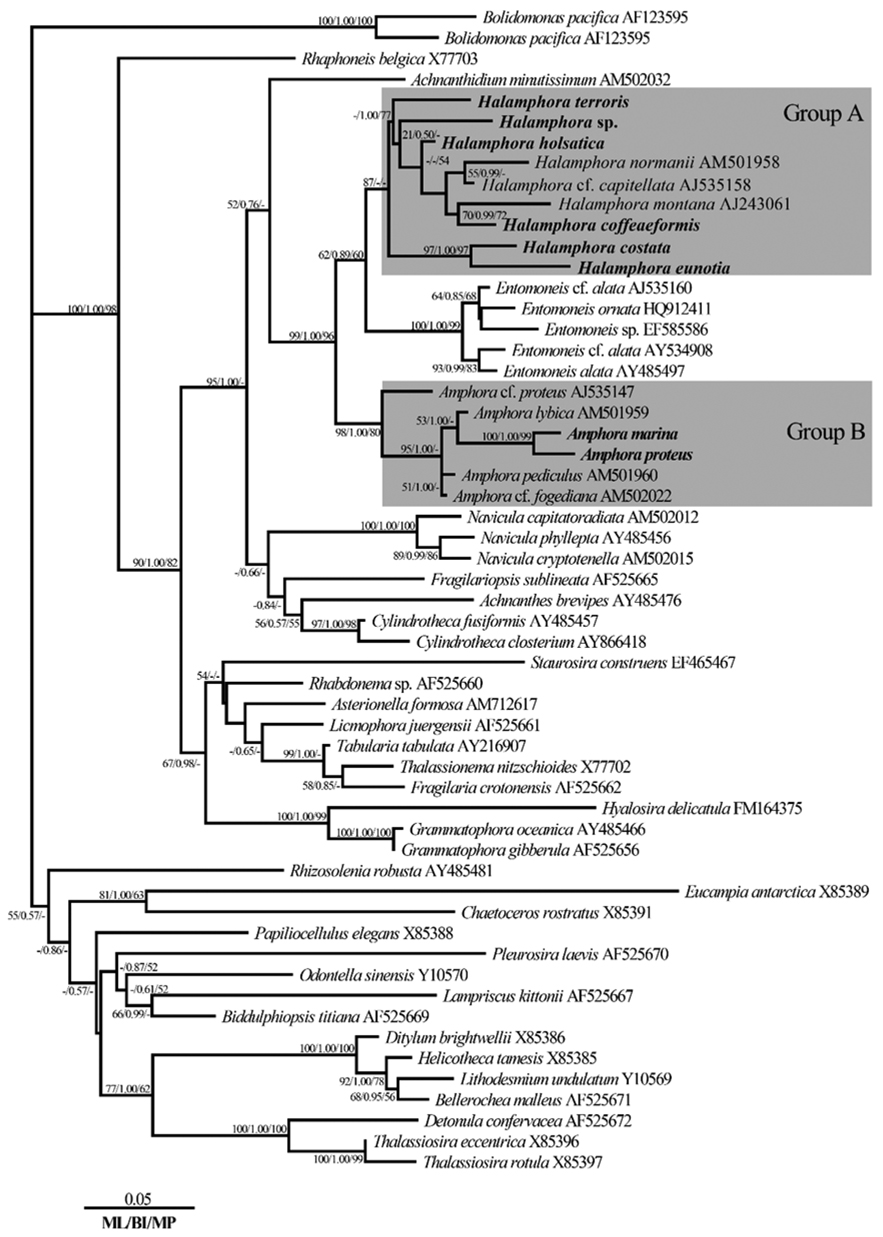
Phylogenetic relationships among the eight species of
Based on an analysis with MrModelTest, the model GTR + I + G was selected for the main results of the comparison of the negative log likelihood (-ln
On the basis of the best-fit model, a phylogenic tree was constructed (Fig. 4). ML scores of the ML tree was calculated to be -ln
The topology was similar for the phylogenetic trees obtained by ML, Bayesian, and MP phylogenetic analysis methods. With respect to the tree in Fig. 4, the genus
>
Morphological characteristics
>
Amphora marina Smith 1857 (
Smith 1857, p. 7, Pl. 1, Fig. 2; Van Heurck 1896, p. 129, Pl. 1, Fig. 14; Péragallo and Péragallo 1908, Pl. XLIV, Figs 15-17; Cleve-Euler 1953, p. 92, Fig. 671; Nagumo 2003, p. 32, Pls 71-75; Levkov 2009, p. 470, Fig. 7.
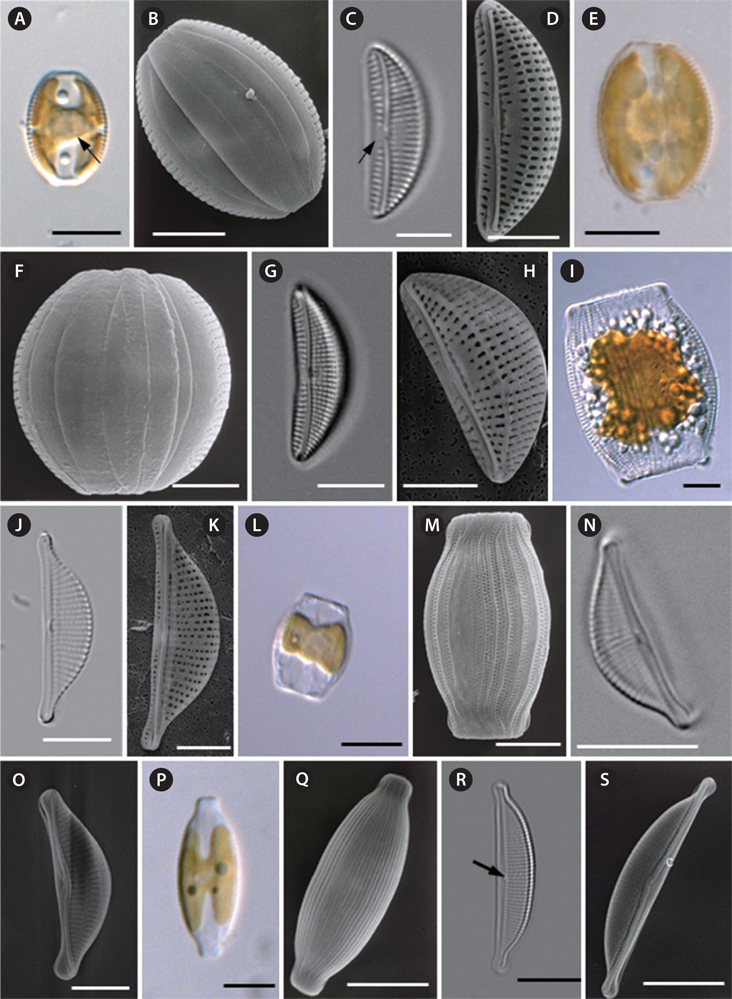
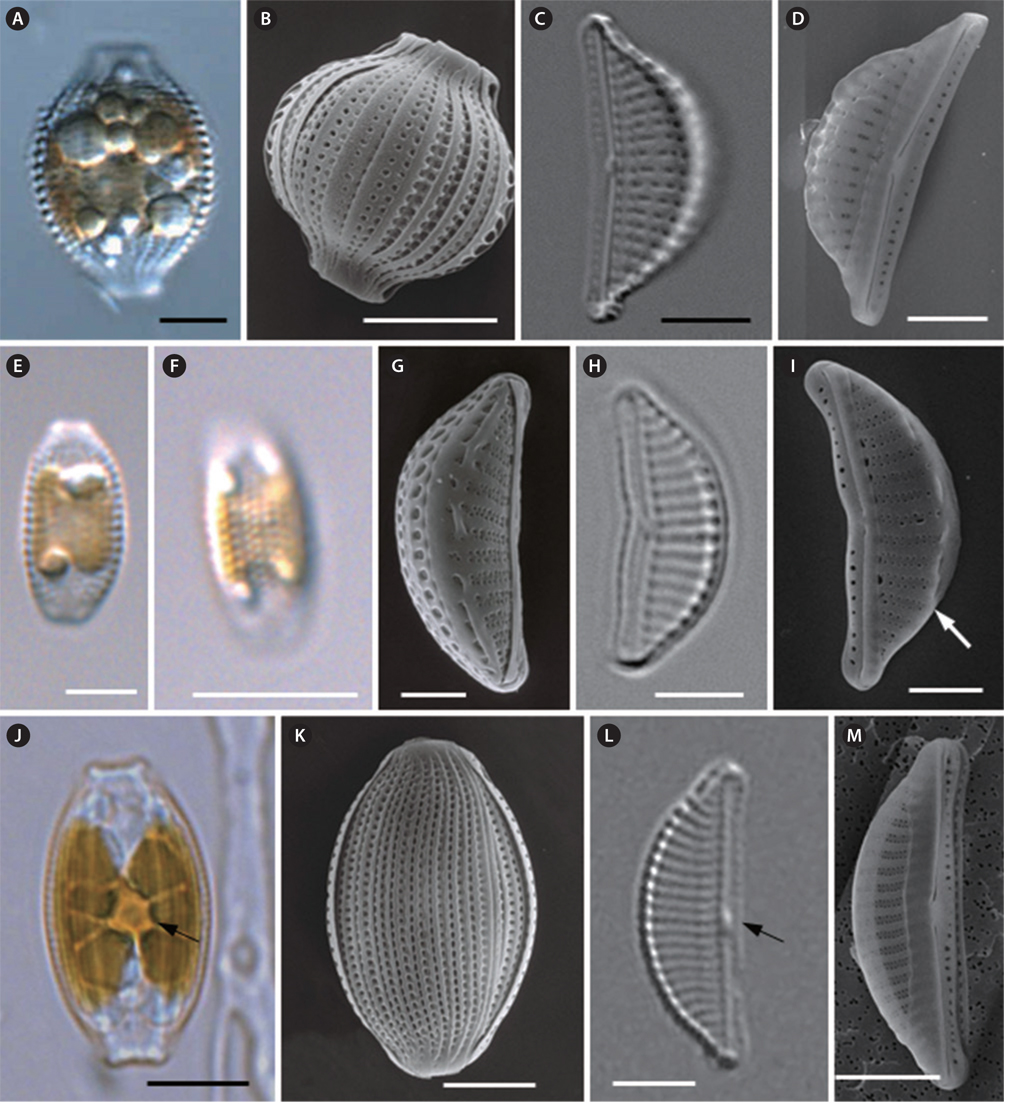
Cells are solitary and chloroplasts are attached to the inner side of the frustule (Fig. 5A). The pyrenoid can be observed clearly at the center of the cell (Fig. 5A). Cells are sessile, but they are usually motile, almost always lying in girdle view. The frustule is elliptical to widely lanceolate with truncated ends (Fig. 5A & B). The girdle bands are not plicate and four girdle bands can be observed (Fig. 5B). Valves are small and lunate, with a convex dorsal margin, a ventral margin that is slightly concave, and with rounded apices (Fig. 5C &D). The valve length is 15.0 to 19.2 µm, and the valve width is 5.2 to 7.9 µm (Table 4). The raphe is well-marked and slightly deflected at the median (Fig. 5C & D). The marginal ridge is not pronounced. The ventral striae are composed of single areolae. The size of the areolae increases towards the mid-valve and they converge toward the ends (Fig. 5C & D). Striae are rather coarsely punctate on the dorsal side and radiate throughout the dorsal side (Fig. 5C), with 17-19 striae present in 10 µm (Table 4). Striae are interrupted by the central area, which is conspicuous at the center of the valve (Fig. 5C & D).
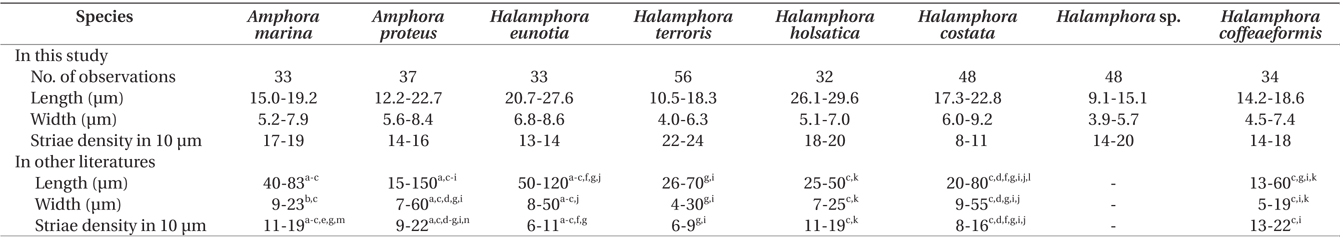
Sizes and striae densities in valves of eight Amphora sensu lato species and records in other literatures
Distribution. Jeju, Korea.
Remark.
>
Amphora proteus Gregory 1857 (
Gregory 1857, p. 518, Pl. 13, Fig. 81; Van Heurck 1896, p. 129, Pl. 24, Fig. 671; Boyer 1916, p. 65, Pl. 15, Figs 5, 6 & 19; Boyer 1927, p. 254; Cleve-Euler 1953, p. 92, Fig. 673; Levkov 2009, p. 548, Figs 1-9, p. 824, Figs 4-6.
Synonyms.
Cells are solitary and chloroplasts are attached to the inner side of frustule (Fig. 5E). Cells are sessile, but they are usually motile, almost always lying in girdle view (Fig. 5E). The frustule is elliptical to widely lanceolate with truncated ends (Fig. 5E & F). The girdle area of the frustule is not plicate (Fig. 5F). Valves are small and lunate, with a very convex dorsal margin, slightly concave ventral margin and rounded apices (Fig. 5H). Valve length is 12.2 to 22.7 μm, and valve width is 5.6 to 8.4 µm (see Table 4). Raphe are well marked and slightly inflexed at the median (Fig. 5G). The marginal ridge is pronounced (Fig. 5H). The ventral margin forms a row of elongate areolae, increasing in length toward the mid-point of the valve (Fig. 5G) and converging toward the ends. Striae with very distinct robust puncta radiate on the dorsal side, with 14-16 striae per 10 µm (Table 4). The central nodule is rather large, with no conspicuous central area (Fig. 5G).
Distribution. Gilcheon Port, Korea.
>
Halamphora eunotia (Cleve) Levkov 2009 (
Cleve 1873, p. 21, Pl. 3, Fig. 17; Van Heurck 1896, p. 129, Pl. 24, Fig. 684; Peragallo and Peragallo 1908, Pl. L, Fig. 17; Cleve-Euler 1953, p. 99, Fig. 687; Levkov 2009, p. 502, Figs 1-12, p. 814, Figs 1-4.
Synonym.
Cells are solitary. Cells are usually motile, almost always lying in girdle view and then appearing sub-rectangular, with the two sides having an arching bulge (Fig. 5I). The girdle area of the frustule is wide and plicate for the punctate girdle bands (Fig. 5I). Valves are semi-lanceolate with a convex ventral margin, and the poles are ventrally deflected with protracted apices (Fig. 5J & K). Valves are 20.7 to 27.6 µm long and 6.8 to 8.6 µm wide (Table 4). Raphe are straight and lie along the ventral edge (Fig. 5J & K). The central terminals of the raphe are slightly bent to the dorsal side and then fold back (Fig. 5K). The central nodule is conspicuous (Fig. 5K). Striae are parallel at the central part and become curved and more divergent toward the ends. Dorsal striae are distinctively punctate (Fig. 5J), with 13-14 striae per 10 µm (Table 4). Ventral striae are hardly visible under LM. Striae are not interrupted by the central nodule area.
Distribution. Geoje, Korea.
>
Halamphora terroris (Ehrenberg) Wang comb. nov. 2014 (
Ehrenberg 1853, p. 526; Cleve-Euler 1953, p. 99, Fig. 689.
Synonym.
Cells are solitary (Fig. 5L). Cells are usually motile, almost always lying in girdle view and then appearing subrectangular with the two sides having an arching bulge (Fig. 5L & M). The girdle area of the frustule is wide and plicate for the punctate girdle bands (Fig. 5M). In each girdle band, there are two rows of punctae. Valves are semi-lanceolate with a convex ventral margin, and poles are ventrally deflected with protracted apices (Fig. 5N). Valve are 10.5 to 18.3 μm long and 4.0 to 6.3 μm wide. From the valve view, the raphe slit is slightly curved and lies along the ventral edge (Fig. 5N & O). The central nodule is conspicuous (Fig. 5N). Externally, the proximal fissures of the raphe are dilated in the central part of the cell and dorsally bent (Fig. 5O). The striae are nearly parallel at the central valve and become divergent toward the ends. Striae are not interrupted by the central nodule area at the dorsal side, and there are 22-24 striae per 10 µm (Table 4).
Distribution. Jeju, Korea.
Remark.
>
Halamphora holsatica (Hustedt) Levkov 2009 (
Hustedt 1925, p. 115, Fig. 4; Hustedt 1930, p. 345, Fig. 633; Cleve-Euler 1953, p. 99, Fig. 688; Sar et al. 2003, Figs 1-13; Levkov 2009, p. 522, Figs 1-11, p. 782, Figs 1-6.
Synonym.
Cells are solitary, usually motile, and almost always lying in girdle view (Fig. 5P). The frustule is elliptical to widely lanceolate, with subrostrate-truncated ends (Fig. 5P & Q). The girdle area of the frustule is plicated for the punctate girdle bands (Fig. 5Q). Valves are semi-lanceolate with a convex dorsal margin and straight ventral margin (Fig. 5R). Valves are 26.1 to 29.6 µm long and 5.1 to 7.0 µm wide (Table 4). The raphe are straight and lie along the ventral edge (Fig. 5R). Viewed under SEM, the raphe at the valve lies near the ventral margin, and both branches of the raphe are straight or nearly straight, forming an obtuse angle of nearly 180˚ (Fig. 5S). The conopeum is welldeveloped on the dorsal side of the valve, straight with a slight widening at the center, and its ends embrace the valve poles (Fig. 5S). Along the transition between the dorsal valve face and mantle, there is no costa (hyaline bar) interrupting the striae (Fig. 5R & S). The central nodule is conspicuous (Fig. 5R). The dorsal striae are parallel at the central part and become slightly divergent at the two ends of the valve (Fig. 5R). Striae are not interrupted by the central nodule area (Fig. 5R), and there are 18-20 striae per 10 µm (Table 4).
Distribution. Pohang, Korea.
>
Halamphora costata (W. Smith) Levkov 2009 (
Smith and West 1853, p. 20, Pl. 30, Fig. 253, Figs 15-17; Wolle 1894, Pl. IX, Fig. 3; Van Heurck 1896, Pl. 24, Fig. 679; Pèragallo and Pèragallo 1908, Pl. L, Fig. 20; Cleve-Euler 1953, p. 99, Fig. 690.
Synonym.
Cells are solitary, usually motile and almost always lying in girdle view (Fig. 6A). The frustule is elliptical to widely lanceolate, with ends broadly protracted into the subrostrate to truncate poles (Fig. 6A). The girdle area of the frustule is plicate for the punctate girdle bands (Fig. 6B). There is a double row of areolae at the girdle band. Valves are semi-lanceolate, with a convex dorsal margin and a nearly straight ventral margin (Fig. 6C). Valves are 17.3 to 22.8 µm long and 6.0 to 9.2 µm wide (Table 4). Raphe are straight and lie along the ventral edge (Fig. 6C). The proximal fissures of the raphe are dilated in the central pores and are dorsally slightly bent (Fig. 6D). The conopeum is well-developed on the dorsal side of the valve (Fig. 6D). The central nodule is conspicuous (Fig. 6C). The striae are parallel at the centrally and become slightly divergent toward the ends (Fig. 6C). Dorsal striae are distinctively punctate and spaced at 8-11 striae per 10 µm, and are not interrupted by the central nodule area (Fig. 6C). Ventral striae may be visible, and are interrupted by the central nodule area (Fig. 6C). The conopeum is fairly well developed on the dorsal side of the valve (Fig. 6D).
Distribution. Gilcheon Port, Korea.
Cells are solitary (Fig. 6E) and usually motile. They are almost always lying in girdle view and appear elliptical with truncated ends (Fig. 6E). Chloroplasts are attached to the inner side of frustule (Fig. 6E). The girdle area of the frustule is plicate for the punctate girdle bands (Fig. 6F). Valves are small and lunate, with rounded apices (Fig. 6G & H). Valves are 9.1 to 15.1 µm long and 3.9 to 5.7 µm wide (Table 4). The raphe slit is nearly straight and lies along the ventral edge (Fig. 6H & I). Both branches of the raphe are slightly inflexed at the median, the raphe has a straight end at the center valve and a curve to the dorsal side at the apices of the valve (Fig. 6I). Along the transition between the dorsal valve face and mantle there is a costa (hyaline bar), but this is not continuous in all cells (Fig. 6I). The costae and striae are observed very clearly and are parallel throughout (Fig. 6H). Ventral striae are poorly visualized under light microscopy in the study specimens. Striae are not interrupted by the central nodule area on the proximal side, and are spaced with 14-20 striae per 10 µm (Table 4). The puncta are alternately spaced in double rows to form striae (Fig. 6G & I). The conopeum is fairly well-developed (Fig. 6I).
Distribution. Gilcheon Port, Korea.
>
Halamphora coffeaeformis (C. Agardh) Levkov 2009 (
Kützing 1844, p. 108, Pl. 5, Fig. 37; Wolle 1894, Pl. IV, Figs 19 & 20; Van Heurck 1896, Pl. 24, Fig. 671; Boyer 1916, p. 65, Pl. 15, Figs 8 & 18; Cleve-Euler 1953, p. 97, Fig. 685; Archibald and Schoeman 1984, p. 86, Figs 1-24, p. 87, Figs 25-29, p. 91, Figs 100-109, p. 92, Figs 110-122, p. 93, Figs 123-134, p. 96, Figs 135-146, p. 97, Figs 147-159, p. 98, Figs 160-162; Krammer and Lange-Bertalot 1986, p. 744, Pl. 151, Figs 1-6.
Synonyms.
Cells are solitary (Fig. 6J), usually motile, and almost always lying in girdle view (Fig. 6J). Single H-shaped chloroplasts are contained in each cell with a conspicuous pyrenoid at the center of the cell (Fig. 6J). The frustule is elliptical to widely lanceolate, with ends somewhat broadly protracted into the subrostrate to truncated poles (Fig. 6J). The girdle area of the frustule is plicate for the punctate girdle bands (Fig. 6K). Valves are semi-lanceolate, with rounded apices (Fig. 6L). Valves are 14.2 to 18.6 µm long, and 4.5 to 7.4 µm wide (Table 4). The central nodule is conspicuous (Fig. 6L). The raphe are straight and lie along the ventral edge (Fig. 6L & M). The costae and striae can be observed very clearly and are parallel at the centrally, becoming curved and more divergent toward the ends (Fig. 6L). Striae are not interrupted by the central nodule area at the proximal side, and are spaced with 14-18 striae per 10 µm (Table 4). SEM images show that the puncta are alternately spaced in double rows to form striae at the valve. Between the striae, the costae are very conspicuous. The ventral striae are denser and consist of only a single row of small linear to circular areolae, which are not uniform in shape, size or arrangement (Fig. 6M).
Distribution. Uljin, Korea.
All eight species studied are clearly distinct from one another, both morphologically and by sequencing analysis. All eight of these species’ morphological characteristics have been compared with previous morphological descriptions; each species is identified.
The
Differences in densities of stria observed in our study and previously may be attributable to measurement methods used by different researchers. Archibald and Schoeman (1984) also discussed this issue. In most publications, the actual site is not given, and one is left to assume that counts were made across the central parts of the valve. To obviate this problem, we emphasize that the striae counts herein are always made along the raphe, as done by Schoeman and Archibald (1976). Striae counts designated as being near the center were made on either side of the central nodule or central area and not across the central nodule.
With regard to
In this study,
ANOVA of similarity and genetic distances showed that the species in subgenus
From our phylogenetic tree, we found that species of
Due to the fact that
More definitive conclusions regarding the phylogenetic taxonomy of