Wettability of a solid surface describes the ability of a liquid to maintain contact with the surface, which is important in the bonding or adherence of two materials [1]. Dependent on both roughness and chemical heterogeneitym, wettability is a very important characteristic in nature as well as in our daily life. Hydrophobicity and hydrophilicity are two principal wettablility conditions. The surfaces of materials are hydrophilic if the water contact angle (WCA, θ) is in the range of 0° ≤ θ < 90° and they are hydrophobic if the WCA is 90° < θ ≤ 180°. Hydrophobicity is observed in nonpolar substances, which tend to aggregate in aqueous solutions and exclude water molecules.
Superhydrophobic materials have recently attracted attention from both academic and industrial circles because of their importance in fundamental research and potential industrial applications such as bio-surfaces, anti-biofouling, transparent and antireflective superhydrophobic coatings, structural color, fluidic drag reduction, enhancing water supporting force, controlled transportation of fluids, superhydrophobic valves, battery and fuel cell applications, prevention of water corrosion and oil-water separation corrosion protective coatings, preventing adhesion of water and snow to windows or antennas, self-cleaning, enhancing buoyancy, and coating films for electronic devices [2-8].
The phenomenon of superhydrophobicity was first studied by Johnson and Dettre [7] in 1964 using rough hydrophobic surfaces. They developed a theoretical model based on experiments with glass beads coated with paraffin and polytetrafluoroethylene telomere, respectively. Barthlott and Ehler [9] studied the self-cleaning property of superhydrophobic micro-nanostructured surfaces in 1977, and they described such self-cleaning and superhydrophobic properties for the first time as the “lotus effect”. The superhydrophobic state of a surface is defined by the contact angle between a water droplet and the surface of a material that has a WCA above 150°. Superhydrophobic surfaces display a self-cleaning effect (water repellent) widely known as the “lotus effect.” Superhydrophobicity can be achieved either by selecting low surface energy materials or by introducing roughness [9-14].
Activated carbons, graphite, carbon fibers (CFs), fullerenes, carbon nanofibers (CNFs) (carbon nanotubes [CNTs], graphite nanofibers [GNFs]), micro-/meso-porous carbon, and, more recently, graphene carbon-based materials have emerged as extremely promising materials for various types of applications owing to their outstanding electronic and optical properties [15- 28]. Recently, carbon-based superhydrophobic surfaces have been fabricated because of their promising applications such as conductive-transparent films, oil-water separation, and electromagnetic- interference-shielding [29-49]. In general, small water droplets on CF fabrics will be up to 120°, but not much greater for a flat surface.
To enhance the hydrophobicity of carbon-based materials, two approaches have been established recently [30-33]. First, research on superhydrophobic carbon-based materials has focused on increasing surface roughness by introducing geometric surface area.
Dip-coatings methods are also often employed to obtain superhydrophobic surfaces. As demonstrated by Nguyen
Most current research on carbon-based materials has focused on their electrical, physiochemical, and mechanical properties [56-58]. In particular, surface properties such as robustness against environmental contamination are critical design considerations if intrinsic properties are to be maintained. Herein, we start by discussing the properties of carbon-based materials with superhydrophobic surfaces provided by emerging methods to create superhydrophobic surfaces with enhanced bulk properties. The recent advances in this field are summarized, including the wetting behavior of water on carbon materials such as CFs, CNTs, graphene, and amorphous carbons, and the formation of hierarchical structures and low surface energy chemical composition, with emphasis on fundamental understanding of related processes. Potential applications in energy, environmental remediation, and thermal management are also discussed [59- 63].
In this paper we discuss the recent theoretical advances in superhydrophobicity, the relation of superhydrophobicity to the more general type of “superphobic” surfaces, manufacturing methods to obtain superhydrophobicity with different kinds of carbon surfaces, and new potential applications of superhydrophobic carbon-based materials such as new energy technology, green engineering, underwater applications such as antifouling, and optical applications.
Superhydrophobicity, as shown by lotus leaves, is an area that has received much interest in the past few years in relation to self-cleaning surfaces and other processes as well as in the design and production of artificial biomimetic surfaces [64- 71]. Fig. 1 shows a structural diagram of the lotus effect [64]. The structures of a lotus leaf, i.e., branch-like nanostructures on top of micropapillae, have been carefully observed by scientists. These microscale-structures can induce superhydrophobic surfaces with large contact angles and low sliding angles. It is believed that this unique self-cleaning property is based on the surface lotus effect illustrated in Fig. 1. The roughness is caused by the microscale papillae and nanoscale tomenta (Figs. 1b-d). To construct superhydrophobic carbon-based material surfaces, it is essential to understand the wetting behavior of water on the surface of the carbons.
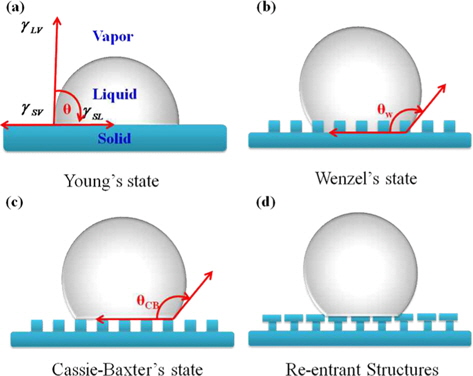
Superhydrophobic surfaces are generally expressed by the contact angle between a water droplet and the solid surface. Wenzel’s early works and later Cassie-Baxter’s work defined the importance of surface roughness and heterogeneity, which are now recognized as key parameters in the wettability of hydrophobic surfaces [65,66]. Wang
Contact angle measurements, as described by Young in 1805, remain the most accurate method for determining the interaction energy between a liquid and solid in a condensed state at the minimum equilibrium distance of solid and liquid. When a droplet of water rests on a surface, the contact angle can be measured at the edge of the droplet (Fig. 1). Fig. 2 shows the liquid droplet wetting behavior of the corresponding theory: (a) Young’s state, (b) Wenzel’s state, (c) Cassie-Baxter’s state, and (d) an example of re-entrant morphology [5,11,14]. More than 200 years ago, Young, identified in a recent biography as “the last man who knew everything” [73], described the forces acting on a liquid droplet spreading on a flat surface (Fig. 1a).
On flat surfaces, Young’s equation defines the contact angle (θ) depending on the solid-vapor, solid-liquid, and liquid-vapor surface tensions, as given in Eq. (1), where
Depending on the level of surface roughness, two different models can be distinguished. The first, shown in Fig. 2b, is Wenzel’s state. The droplet maintains contact with the surface and fills the asperities, and the surface area associated with the contact angle is increased by the roughness factor r.
Wenzel’s state is described by Eq. (3).
In Eq. (3), r is equal to the ratio between the actual surface area of the rough surface and the projected (apparent) area, where the non-dimensional surface roughness factor r > 1. r emphasizes the effect of the surface chemistry determined by the term cos
If the droplet is suspended on the surface asperities and the liquid does not penetrate the protrusions of the surface features, then this case belongs to Cassie-Baxter’s model. In this model, the apparent contact angle is the result of all contributions of different phases [82]. In Eq. (4),
From these two theories, it can be found that surface topography can enhance surface wettability on solid surfaces, whether they are hydrophobic or hydrophilic. This finding guides us to tune the surface wettability by controlling surface geometrical structures independently of the chemical composition [82-87]. On the other hand, the surface morphology was found to be an important parameter and more precisely “reentrant” structures are required to obtain such a superhydrophobic surface (Fig. 2d). These morphologies induce a negative Laplace pressure difference because they introduce a transition in the liquid-vapor interface from concave to convex, inducing a higher energy barrier between Wenzel’s states and Cassie-Baxter’ states [86,87].
2.2. Modification of roughness of low surface energy materials
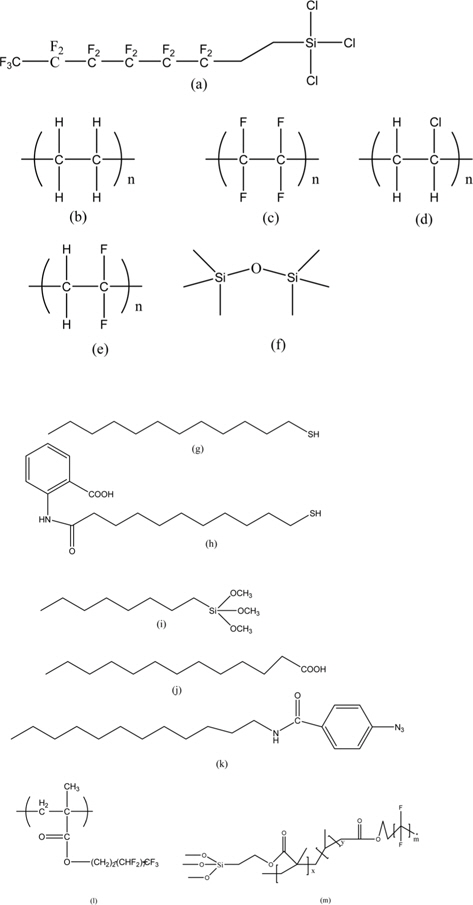
Based on the general contact angle theory of Wenzel, two main approaches have been developed to generate superhydrophobic surfaces. One is increasing the surface area on a microscopic scale of low surface energy materials, as described above in Section 2.1; the other is to fabricate a suitable surface roughness with certain materials and subsequently modify the as-prepared surface with low surface energy materials [88]. The latter method is no longer limited to low surface energy materials, and it can be extended to the fabrication of hydrophobic surfaces for many systems. Various low surface energy materials have been developed to modify microscopic scale surfaces to form superhydrophobic surfaces. Fluorinated compounds, silane, and long alkyl chain fatty acids are typical low surface energy compounds, and have the ability to endow various substrates (SiO2, TiO2, Al2O3, metals, polymers etc.) with high hydrophobicity. Fig. 3 shows various surface reactive molecules for low-surfaceenergy modifications [88-90].
The surface energy is generally defined as the work required to build a unit of area of a given surface, using the sessile drop technique [91,92]. Numerous such theories have been developed by various researchers. These methods differ in several ways, in terms of derivation and convention for example. Most importantly, they differ in the number of components or parameters. Simpler methods containing fewer components simplify the system by converging the surface energy values into one number, whereas strict methods using more components are derived to distinguish various components of the surface energy. In addition, the total surface energy of solids and liquids depends on the different types of molecular interactions, such as the London dispersive and the polar or acid/base interactions, and is considered to be the sum of these independent components [92].
In the early 1960s, Fowkes [93,94] introduced the concept of the surface free energy of a solid. The total surface free energy can be divided into the London dispersive and specific (or polar) components [93-95].
where
where θ is the contact angle of a liquid droplet in the solid state. As reported in our previous work, the specific component
CFs have attracted considerable interest due to their electrical properties, thermal conductivity, and high strength, lending outstanding behavior in practical applications [96-101]. In many cases, the CF surface properties are a crucial factor in their performance. Their specific surface area, however, is not sufficiently large for them to serve as ideal electrochemical materials compared to other carbon nanomaterials. Superhydrophobicity is an effect where roughness and hydrophobicity combine to generate unusually hydrophobic surfaces [81,100-105]. Because CFs are intrinsically hydrophobic, surface treatment is usually required to induce a hydrophilic state prior to their practical use [106-108]. The pristine hydrophobicity of CFs hinders their widespread utilization, and research on potential applications of this material generally has been limited.
Bliznakov
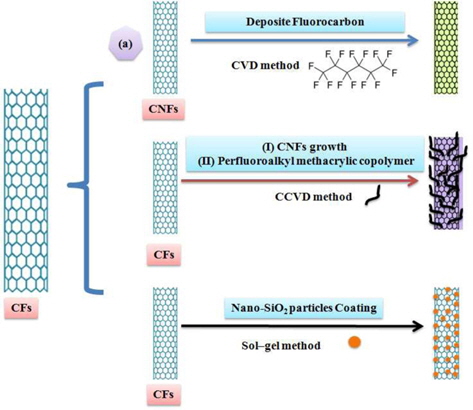
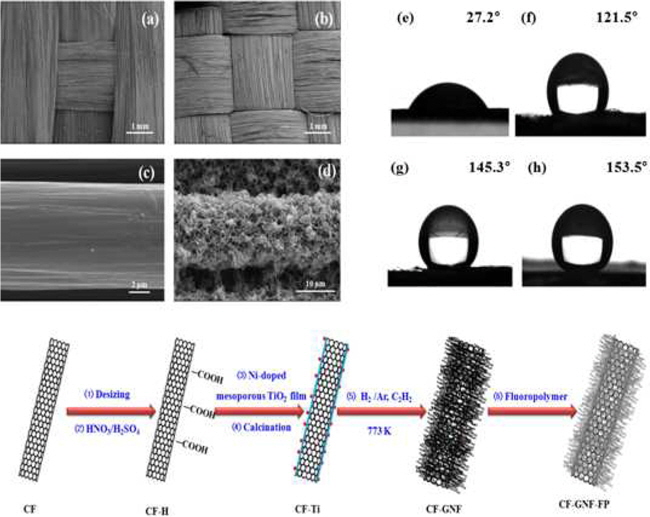
The aforementioned method for the growth of CNTs on CF surfaces has some drawbacks. For example, at the high synthesis temperature, the metal catalysts easily aggregate into large particles and form different kinds of carbon nanomaterials. In addition, these processes require purity to attain adequate superhydrophobic and electrochemical properties [104]. Park
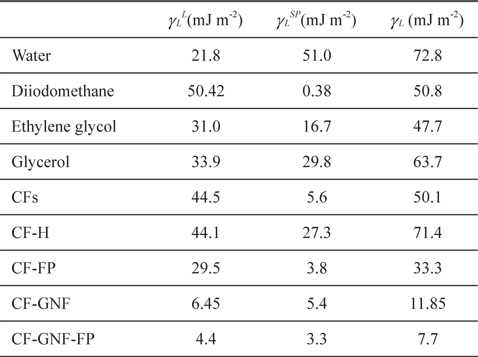
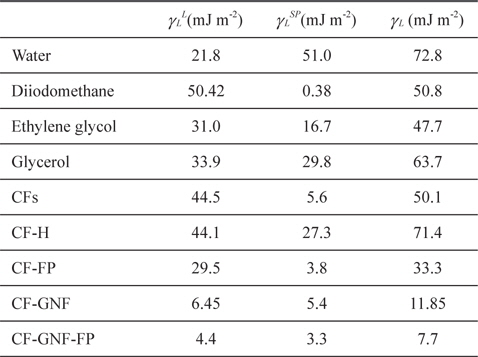
Dispersive (γ SL), polar (γSSP), and surface free energy (γS) components of wetting liquids used in Park’s work: pristine CFs, oxidized CF-H, Ni-doped mesoporous TiO2 film coated CF, fluoropolymer coated CFs, GNF coated CFs, and fluoropolymer coated CF-GNF (unit: mJ m?2)
Lu et al. [110] synthesized CFs and SiCO/carbon composite fibers with average diameters of 120 and 163 nm, respectively, by electrospinning 7 wt% PAN and 5/7 wt% polyureasilazane (PUS)/ PAN in dimethylformamide (DMF), respectively, followed by cross-linking, stabilization, and carbonization at temperatures up to 1000℃. The SiCO/CFs exhibited dual superhydrophilicity (absorbing 873% water) and superoleophilicity (608% decane absorption). The electrochemical properties determined by cyclic voltammetry show that the SiCO/CFs possess better capacitance behaviors than CFs.
Seo et al. [111] fabricated nm-scale carbon structures on CFs with micrometer-scale thickness using the CVD method, where Ni nanoparticles were used as catalysts of nanostructure growth. Polydimethylsiloxane (PDMS) thin films were used to provide hydrophobic surface properties. To adjust the pH value of the aqueous solutions, HCl and NaOH were used for acidic and basic solutions, respectively. For both cases, the initial contact angle measured immediately after dropping 3 μL of liquid droplet was higher than 170°. With increasing contact time to 30 min, the contact angles were almost constant. This implies that the superhydrophobicity of PDMS-coated surfaces can be sustained in a corrosive environment.
Qiu
4. Superhydrophobicity on CNTs
CNTs, especially those with a cylindrical nanostructure, are of immense research interest for their outstanding behavior in practical applications such as nanotechnology, electronics, optics and other fields of materials science and technology [43,63,90,113-119]. However, in many cases, CNTs wettability and dispensability are crucial factors in their performance; example applications include reinforced polymer composites, templates, sensors, catalysts, electrode, etc. To date, many investigators have endeavored to fabricate CNTs having a superhydrophobic surface.
Previous studies have mostly focused on aligned CNTs films [43,90,117-119]. Zhu
Hong and Uhm [118] prepared superhydrophobic CNTs by low-pressure CF4 glow plasma to provide roughness and fluorination to CNTs. The total surface free energy of CNT powder treated by CF4 plasma for 20 min was calculated to be drastically decreased from 27.04 to 4.06 × 10−7 mJ/m2. Superhydrophobic CNT powders were prepared by employing CF4 glow discharge plasma to provide roughness and fluorination to CNT powders and water droplets bouncing on CNT powders were observed.
Luo
It was recently reported that vertically aligned multi-walled CNT (VACNT) films produced by different techniques can present a hydrophobic character [120]. In particular, CO2 laser irradiance was used to modify the CNT surface by decreasing the polar component of the surface energy. Ramos
Stimuli-responsive smart surfaces with dynamically tunable wettability have recently received special attention because of their potential applications [121-123]. Yang
Most of the current strategies for fabricating durable hydrophobic surfaces can provide contact angles close to 170° coupled with icephobicity [32,124]. Zheng
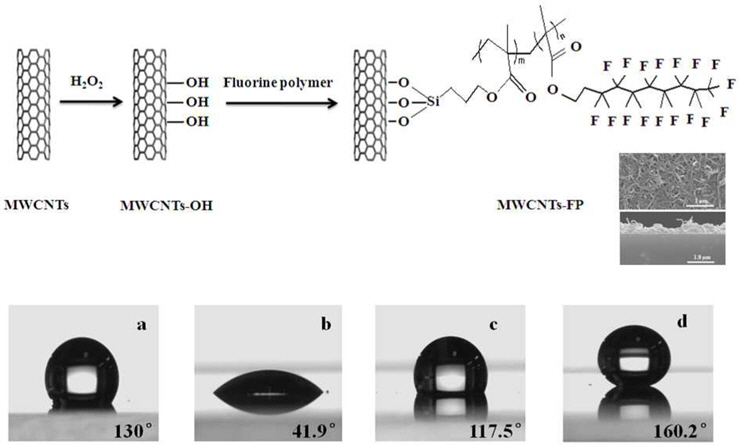
Recently, superhydrophobic transparent conductive films have attracted considerable interest due to their importance in fundamental research and potential industrial applications [48,125-127]. Meng and Park [125] prepared multi-walled CNT (MWCNT) thin films on glass substrates. The prepared films showed transparent, conductive, and superhydrophobic properties. MWCNTs were dispersed in FP solutions for modification of their surface by grafting a FP (Fig. 7). A dip-coating process was used to prepare the films at a continuous speed and different numbers of coatings were applied. Fig. 7 shows schematic diagrams illustrating the changes of the contact angle and the surface properties of the film. As shown in this figure, the water droplet retains an ellipsoidal shape on the MWCNTs with a contact angle of 130°, suggesting the raw MWCNT materials have a hydrophobic character (Fig. 7a). The sharp decrease of the CA from 130° to 41.9° for MWCNT-OH (Fig. 7b) originates from the hydrophilic properties of the fully H2O2 treated surface. This is due to an increase of hydrophilic functional groups (–OH, C=O, and O=C–OH) during the H2O2 oxidation process [21]. The CA of glass increases to 117.5° after being coated with FP, and this is ascribed to the surface of the glass being coated with densely packed –CF3 groups (Fig. 7c). The CA of MWCNTs-OH after treatment with the FP increased to 160.2°, and the surface showed superhydrophobicity (Fig. 7d). The CA was 160.2° even at a transmittance of 83.5% (at 550 nm) and a sheet resistance of 1.38 × 104 Ω sq−1. This clearly indicates that the networks comprised of MWCNTs increase the conductivity while those containing the FP did not affect the conductivity of the films.
Tang
5. Superhydrophobicity on Graphene
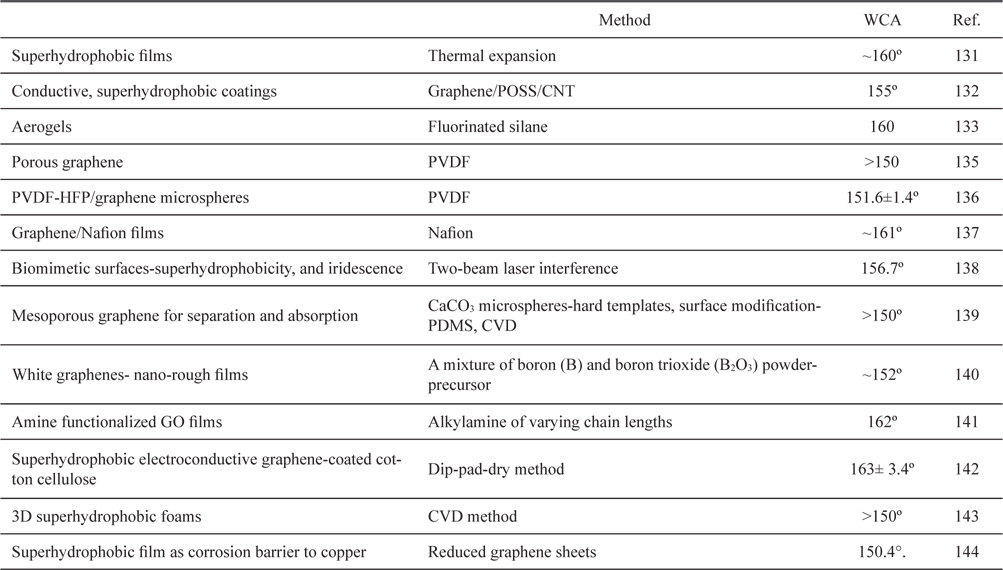
Graphene is a single-atom-thick sheet composed of sp2-hybridized carbon atoms and it exhibits many intriguing properties [48,127-130]. A perfect graphene nanosheet is hydrophobic, but surface treatment or introduction of various functional groups or additional components will remarkably vary its surface properties. Graphene has aroused strong interest in the context of exploring novel functional superhydrophobic surfaces [30]. Several experimental and modeling studies have focused on exploiting micro-scale surface roughness to engineer superhydrophobic graphene. Several research groups have fabricated superhydrophobic graphene surfaces using an irregular stack of graphene oxides (GO) prepared by chemical oxidation of graphite, and pertinent results are shown in Table 2 [131-144].
[Table 2.] List of graphene-based superhydrophobic surfaces
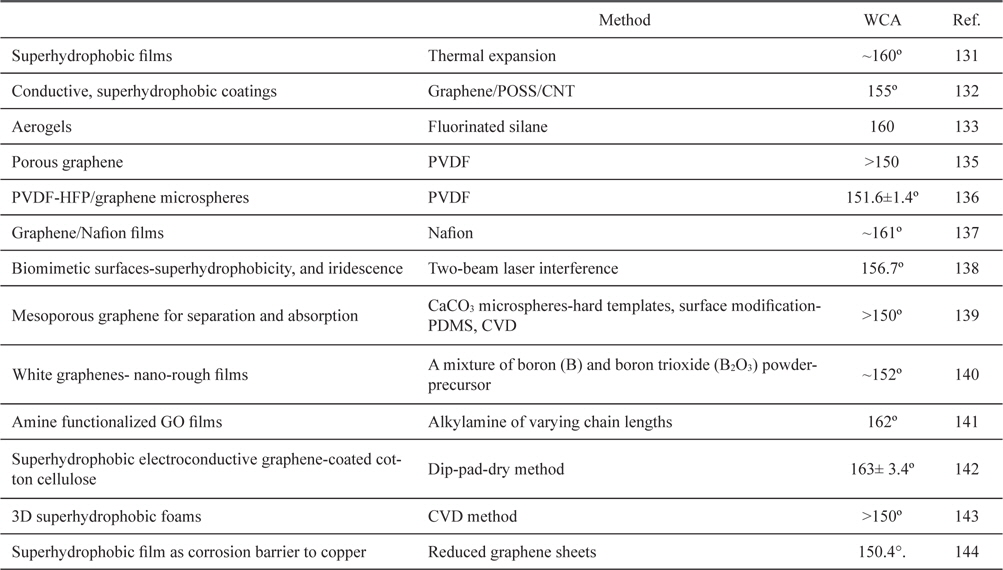
List of graphene-based superhydrophobic surfaces
Rafiee
Lin
As shown in Fig. 3, fluorinated polymers are commonly used as low surface energy materials; for example, polyvinylidene fluoride (PVDF) is used to produce superhydrophobic materials [134-136]. Zha
Usually, the micro-/macro-scale surface structures of functional films strongly affect the critical properties, such as wettability, and they should exhibit super water repellency with a WCA larger than 150° for self-cleaning and antimicrobial surfaces. Choi and Park [137] demonstrated superhydrophobic thin films of graphene-based materials induced by a hierarchically petal-like structure. The superhydrophobic graphene/Nafion nano hybrid films were prepared by controlling the structures with respect to the chemical composition from an interpenetrating networked and compactly interlocked structure (specific surface area of 9.56 m2/g) to a hierarchical petal-like, porous structure (specific surface area of ~ 413 m2/g). The hybrid films revealed a petal-like, porous structure with hierarchical roughness, where microscale roughness was produced in the lateral direction of the hybrid sheets while nanoscopic roughness was created on the edges of the hybrid sheets. The surface morphologies of the hybrids were changed with respect to the amount of Nafion and their CAs increased from ~97° to ~161° with increasing Nafion content.
Shanmugharaj
Singh
6. Superhydrophobicity on Other Carbons
There has been a continuous growth of interest throughout the world in synthesizing porous carbons to fabricate superhydrophobic films, in addition to CFs, CNFs, CNTs, and graphene. A typical method to control the wettability of a solid is surface modification with low surface energy materials. Superhydrophobic surfaces can then be fabricated successfully with micro- or nanostructured surfaces. As an environmentally benign and economically viable optoelectronic device material, superhydrophobic amorphous carbon films are of interest in various applications [35,145-147].
Zhou
Li
Banerjee
Carbon is a versatile material due to its porosity, conductivity, thermal conductivity, wide operating potential range, high chemical stability, and reasonable cost. In this review, we have presented different techniques for the preparation of superhydrophobic carbon-based materials such as CFs, CNFs/CNTs, graphene, amorphous carbons, and porous carbons. These superhydrophobic carbon-based materials hold great promise for the development of various industrial products. Overall, there are two traditional methods to control superhydrophobic surfaces: 1) surface micro-scale roughness; and 2) low surface energy materials treatment. Several technologies that can improve surface roughness and decrease surface energy have been developed, including thermal treatment, chemical modification with –(CH2)n–CH3 or (CF2)n –CF3 groups containing materials, CVD method, plasma treatment, an electro-spinning. From a commercial point of view, carbon-based superhydrophobic materials can be applied to smart surfaces with tunable wetting behavior in different stimuli-responsive environments. They have attracted considerable attention for applications in self-cleaning surfaces, anti-adhesive coatings, biosensors, microfluidics, and many other areas.



![Lotus effect: (a) lotus leaf and beads; (b) scanning electron microscopy image of micropapillae present on the surface of a lotus leaf; (c) image of a water droplet on a lotus leaf (a); (d) structural diagram of micro- and nanostructure of a single micropapilla [43].](http://oak.go.kr/repository/journal/13522/HGTSB6_2014_v15n2_89_f001.jpg)