Because graphite has an anisotropic crystal structure and boasts far better heat resistance, corrosion resistance, electric conductivity, high-temperature strength, and lubricity than other materials, it is used in diverse fields. These include use in high-temperature structural materials such as electrodes, carbon brushes, mechanical seals and special mechanical parts [1-3]. When graphite with such an anisotropic structure is formed using the cold isostatic pressing method, graphite particles are arranged in a disorderly manner, exhibit isotropy, have a dense structure, and are high in density and strength. In addition, because the green body has no directionality, its physical and electrical characteristics are uniform in all directions (isotropic) [4,5].
In the general production process for an isotropic, synthetic, graphite block; petroleum- or tar-pitch-derived coke powder is evenly mixed with a binder such as pitch or resin, after which it is formed and then carbonized. At this stage, because of the volatilization of the binder due to carbonization, pores are generated inside the green body, and the green body is impregnated with pitch or resin to fill the pores. After filling the pores by several impregnation cycles, the green body is graphitized by thermal treatment at 2500℃ or above [6-8].
After being produced through such a complicated process, the isotropic, synthetic, graphite block is processed for a specific usage, and a large amount of scrap (ISGS) is generated. The graphite scrap generated during the process is added to molten iron to increase the carbon content at steel mills or used to produce graphite-added refractory materials. In particular, when producing carbon-magnesite bricks, approximately 15-25% graphite scrap is added. However, the overall amount of graphite scrap used is not large, and most of it is discarded [9-11].
Consequently, the present study sought to produce isotropic bulk graphite using ISGS. Phenolic resin was used as the binder, and changes in the density and porosity were measured after a single impregnation of the recycled isotropic bulk graphite. In addition, using X-ray diffractometer (XRD), the degrees-ofalignment (Da) and microstructure according to the direction of compression molding were measured, and the anisotropy ratio was obtained from the Da. The results of the present study were used to evaluate the potential of producing isotropic bulk-graphite from ISGS, and to provide data for future production of highdensity, isotropic bulk-graphite.
2.1. Raw materials and preparation
The raw powder used in the present study was ISGS remaining after processing of isotropic bulk graphite for machining [12]. The particle size of the raw powder was measured using a particle size analyzer (Malvern Ins. GB/Mastersizer 2000). The binder was phenolic resin produced by Kangnam Chemical Co., Ltd. The mixture of the raw material and the binder was subjected to uniaxial press-forming at a pressure of 300 MPa, thus producing a green body with a diameter of 10 mm. The green body thus formed was carbonized for one hour at 700℃ in nitrogen gas. The pores generated by the carbonization process were re-carbonized after being resin-impregnated one time.
2.2. Density and porosity measurement
Changes in the density and porosity of the recycled bulk graphite, before and after impregnation, were measured using the Archimedes method (ISO 18754: 2003). The density measured in the present study is volumetric density, which includes both open and closed pores; and the porosity was obtained from measurement of the volume of open pores. Here, ‘open pores’ refers to penetrating pores and ink-bottle pores through which fluids can infiltrate [13,14].
2.3. Observations of microstructure
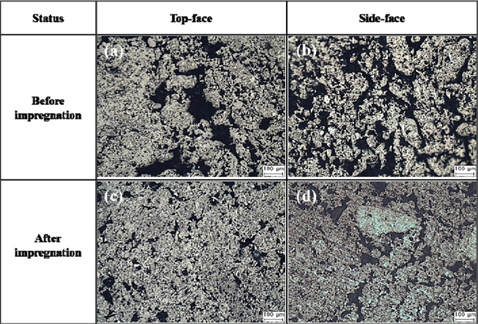
The bulk graphite produced was ground using sandpaper (#1200-#2400) and ultimately micro-ground at 0.25 μm. An optical microscope (Nikon Eclipse, LV150) was used to examine the microstructure of the bulk graphite before and after impregnation. One side perpendicular to the direction of compression molding (henceforth “top-face”), and one side parallel to the direction of compression molding (henceforth “side-face”), was examined.
To confirm the Da of the recycled bulk graphite, an XRD (SWXD, X-MAX/2000-PC, Rigaku) analysis was conducted. The wavelength (Cu-Kα1) of the X-ray target used was 1.5406 Å, and XRD spectra were obtained using the 2θ continuous scanning method at a scanning rate of 1°/min, within a scanning range of 10-60°. Using the XRD data, the top-face and the side-face were measured, and the Da values were compared. The Da values were calculated according to the following equation, using the values of relative strength divided by the height at the (100)-peak and at the (002)-peak. Below, I002 and I100 are the height at the (002)-peak and the (100)-peak, respectively [15,16]:
Da = I100/(I100 + I002)
In addition, the anisotropy ratio was obtained from the Da ratio (DaTop/DaSide) of the top-face and the side-face [17-19].
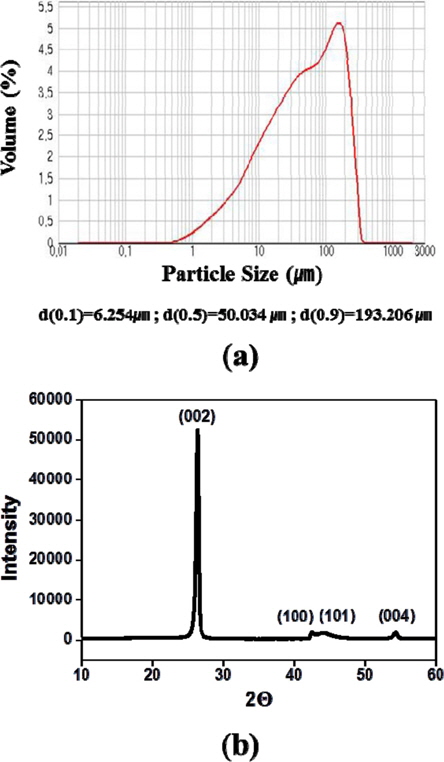
Fig. 1 shows the results of the particle-size analysis of ISGS and the XRD spectra. The average particle size in the raw powder was approximately 50 μm. In the XRD spectra, the strength of the (002)-peak is very great, and the (100)-peak and the (101)-peak observed at the 2θ positions of 42.22 and 44.39 (JCPDS-ICDD #411487) are clearly distinguished. This shows the outstanding crystallinity of ISGS.
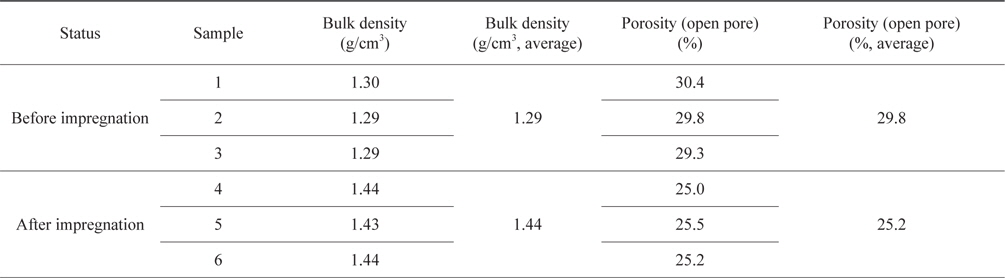
Table 1 shows the density and the porosity of the bulk graphite before and after impregnation, of the three samples measured. The density of the pre-impregnation bulk graphite and the post-impregnation density were 1.29 g/cm3 and 1.44 g/cm3, respectively, thus exhibiting an increase of 11.1% after impregnation. The pre-impregnation porosity was 29.8%, and the postimpregnation porosity was 25.2%, respectively, thus showing a decrease of 4.6%. This decrease in the porosity can be seen as the result of infiltration and impregnation of resin into the open pores. Consequently, if the iteration of impregnation is increased, it is expected that high-density bulk-graphite using recycled ISGS can be produced.
[Table 1.] Result of bulk density and porosity (open pore) measurement
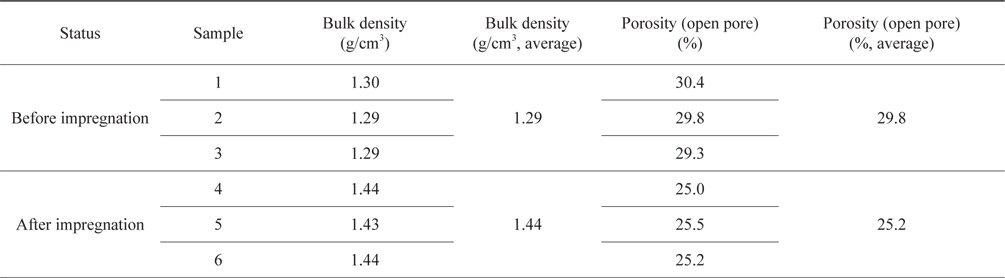
Result of bulk density and porosity (open pore) measurement
Fig. 2 shows the microstructure of the bulk graphite before and after impregnation. In the microstructure of the top-face and the side-face, it was possible to observe the directionality of the particles. Consequently, the possibility of producing isotropic bulk graphite using ISGS was confirmed. It was also possible to confirm a decrease in the pore distribution of the microstructures after impregnation and, as indicated by the data on density and porosity, to confirm an impregnation effect.
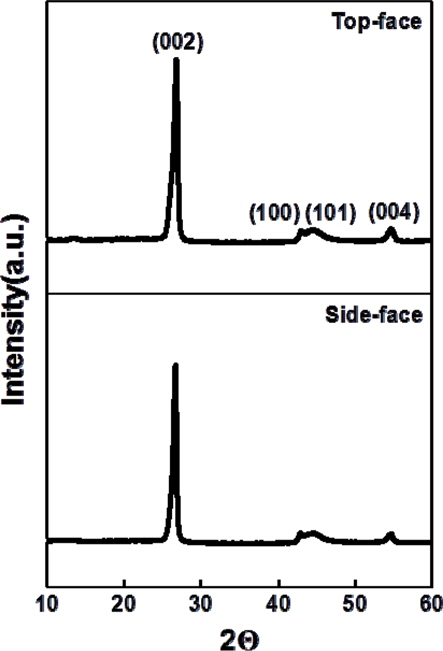
Fig. 3 shows the top-face and side-face XRD spectra of bulk graphite. It clearly shows the (002)-peak, and a distinction between the (100)-peak and the (101)-peak was confirmed. Differences in the XRD spectra between the top-face and the side-face were not observed. Table 2 shows the Da and the anisotropy ratio from the strength of the (002)-peak and the (100)-peak of the XRD spectra. The Da values of the top-face and the side-face were 0.071 and 0.063, respectively, and the difference between them was 0.008, thus confirming that there was no alignment in a particular direction. This also agreed with the earlier results of study of the microstructure. In addition, the anisotropy ratio calculated with the Da of the top-face and the side-face was 1.13, thus confirming the production of comparatively good, new, isotropic bulk-graphite [20].
[Table 2.] Degree of alignment and anisotropy ratio from X-ray diffractometer

Degree of alignment and anisotropy ratio from X-ray diffractometer
The present study sought to produce isotropic bulk graphite using recycled ISGS, and the characteristics of the bulk graphite thus produced were studied, leading to the following conclusions:
The density and porosity of the new bulk graphite produced were 1.29 g/cm3 and 29.8%, respectively. After a single resin impregnation, the density rose to 1.44 g/cm3 (11.1% increase) and the porosity fell to 25.2% (4.6% decrease).
According to the results from examination of the microstructure, the pores in the new bulk graphite were confirmed to decrease after impregnation, but directionality of particles exposed on the top-face and side-face was not observed.
According to the results from calculations obtained using XRD spectra, the Da values were 0.071 for the top-face and 0.063 for the side-face: a difference of 0.008, and the anisotropy ratio was 1.13: confirming that the newly produced bulk graphite was isotropic.
Consequently, the present study confirmed that it is possible to produce isotropic bulk graphite using recycled ISGS. It is expected that it will be possible in the future to produce highdensity, isotropic bulk-graphite by increasing the number of impregnation cycles.