Perfluorooctanoic acid (CF3(CF2)6CO2H, PFOA) is a synthetic perfluorinated carboxylic acid used as a polymerization aids in the production of fluoropolymers.1 Fluoropolymer products are widely used in multiple commercial products including Teflon®, which is used for the coating of cookwares and food packaging, and Gore-Tex® fabrics. Ubiquitous uses of fluoropolymers in our daily life raises increased concerns on human exposure to perfluorochemicals (PFCs) including PFOA and their potential toxicities.2-5 PFOA is persistent in the environment and its exposure is known to be potentially related with increased risks of carcinogenesis and endocrine disruptions. Potential sources of PFOA exposure to human include food and food packaging, drinking water, indoor and outdoor air and dust. Considering relatively long half-life of PFOA elimination from serum (4.4 years),6 bio-monitoring studies of PFOA in human blood serum is needed to evaluate the exposure and related health risks. Previous studies showed several ng/mL of PFOA present in human blood serum,7 although up to 100 mg/mL level has been reported for an occupationally exposed case.8 It should be noted that a survey study of PFOA in human serum showed exclusively higher mean concentration for Korean,9 which implicates the importance of further clinical investigations related with PFOA exposure of Korean.
Wide range of PFOA level in blood serum among populations requires optimized and validated analytical methods including robust sample preparation with minimal matrix effect and maximum recovery of the analyte. Several analytical methods based on the liquid chromatography-mass spectrometry (LC-MS) including various sample preparation have been reported for perfluorinated organic acids.10-12 In this study, a solid phase extraction (SPE) and LC-MS/MS based method has been optimized specifically for hydrophilic-lipophilic balance (HLB) SPE. The optimization of the SPE method for robust quantitative analysis of PFOA in serum was carried out by using a stable isotope labeled surrogate standard, 13C8- PFOA.
Perfluorooctanoic acid, ammonium acetate, acetic acid and formic acid were purchased from Sigma Aldrich (St. Louis, MO, USA). As an isotope-labeled surrogate standard, 13C8-perfluorooctanoic acid (13C8-PFOA) was obtained from Cambridge Isotope Laboratories, Inc (Tewksbury, MA, USA). HPLC grade acetonitrile and methanol were from Burdick and Jackson (manufactured by SK Chemical, Ulsan, Korea). Deionized water was prepared in-house using an Alpha-Q ultrapure water system (Merck Millipore, MA, USA). Fetal bovine serum (FBS) was obtained from Lonza (Basel, Switzerland).
>
Test sample preparation for evaluation of SPE methods
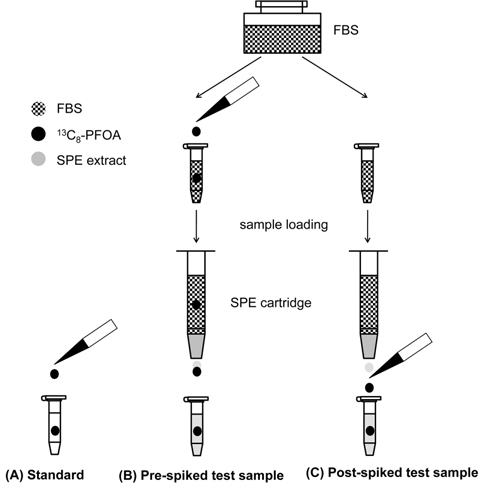
Stock solution of 13C8-PFOA was prepared as 5 mg/mL of methanolic solution. For the evaluation of effective recovery for SPE procedure with additional information on the related process efficiency and matrix effect, three test solutions were prepared (Figure 1). The 50 ng/mL standard solution of 13C8-PFOA (A) was prepared by directly diluting an aliquot of the stock solution in methanol (Figure 1a). A test sample with 5 ng of the surrogate standard per 100 μL of FBS was prepared by adding appropriate amount of the stock solution of 13C8-PFOA in FBS (pre-spiked FBS sample). Three subsamples were taken from the pre-spiked FBS to evaluate reproducibility of SPE process. The test solution B was prepared by dissolving dried eluent obtained from SPE of 100 μL pre-spiked FBS sample using 100 μL of methanol (Figure 1b). Post-spiked test sample was prepared by spiking 5 ng of 13C8-PFOA in the SPE eluent of a 100 μL intact FBS. Final reconstituted solution of the dried SPE eluent mixture using 100 μL of methanol was used as the test solution C (Figure 1c).
The effect of prior protein precipitation before SPE of FBS was evaluated using two different sample pretreatment methods. For protein precipitation, 300 μL of methanol with 1% formic acid was added to 100 μL of FBS test sample. After 10 minutes of sonication, 20 min vortexing and 5 min of centrifugation at 13,200 rpm, the supernatant was transferred to a new Effendorf tube and added with 1.2 mL of deionized water. For sample preparation without protein precipitation, 900 μL of aqueous 1% formic acid was added to 100 μL of FBS test sample. After 10 min sonication and 20 min vortexing, the mixture is direct used for SPE.
>
Optimization of SPE condition
HLB SPE cartridges (cartridge volume 1 cc with 10 mg of resin) were purchased from Waters (Milford, MA, USA). It was pre-conditioned using 1 mL of methanol and 1 mL of 0.1 % aqueous formic acid, then the pre-treated sample was loaded on the cartridge. Three different washing and elution procedures were tested to optimize the SPE efficiencies. The first method (SPE condition 1) followed the previous method10 with a little modification, which included washing condition using 1 mL of 0.1 M formic acid, 2 mL of water:methanol mixture (1:1) with 0.1 M formic acid, followed by 0.3 mL of 1% ammonium hydroxide. Then, the analytes were eluted using 1 mL of acetonitrile with 1% ammonium hydroxide. Because of the potential loss of PFOA with high organic wash in the SPE condition 1, new conditions with higher aqueous wash were investigated. In the second condition (SPE condition 2), sample loaded cartridge was washed using 2 mL of water:methanol mixture (8:2) with 0.1 M formic acid and eluted with 1 mL of acetonitrile. The final method (SPE condition 3) used 2 mL of water:methanol mixture (8:2) with 0.1 M formic acid for washing followed by 1 mL acetonitrile with 1% ammonium hydroxide for elution. All SPE eluents were dried and reconstituted with 100 mL of methanol for LCMS/ MS analysis.
>
Analysis of PFOA and 13C8-PFOA by LC-MS/MS
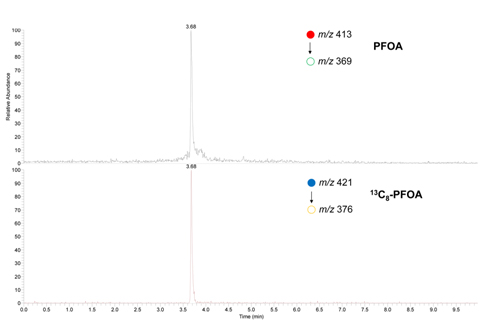
An Agilent 1200 HPLC system (Agilent, Palo Alto, CA, USA) interfaced to a Thermo LTQ linear ion trap MS with electrospray ionization (Thermo, San Jose, CA, USA) was used for the analysis of PFOA and 13C8-PFOA. The LCMS/ MS condition was established starting from the previous LC and MS/MS methods.11-12 In brief, Kinetex XB C18 column (2.1 mm ID, 50 mm in length, 2.6 μm particles with 100Å pore size) from Phenomenex (Torrance, CA, USA) was used with a guard column. Five microliter of the test solution was injected onto the column. Analytes in the injected sample were eluted through the column using a gradient elution of mobile phase A (20 mM ammonium acetate, pH 4) and B (Acetonitrile) at a flow rate of 0.5 mL/ min. The initial elution started from 30% B and maintained for 0.5 min, then increased to 100% B for 6.5 min, and finally maintained 100% B for 3 min. For ESI, 3.5 kV of spray needle voltage and 300℃ of heated capillary temperature were used in the negative ion mode. The selected reaction monitoring (SRM) was used for the quantitative analysis of PFOA and 13C8-PFOA using the MS/MS fragmentation transitions of m/z 413 to m/z 369 and m/z 421 to m/z 376 for PFOA and 13C8-PFOA, respectively. The collision energy for SRM was adjusted to optimize the sensitivity. Each LC-MS/MS sample run was triplicated for statistical data analysis.
>
The optimization of LC-MS/MS analysis condition
The present study has been carried out to develop an optimized SPE-based sample preparation method specifically adapted to follow-up LC-MS/MS analysis as well as LC-MS/MS method itself for the analysis of PFOA in biological fluids. The LC separation method was optimized based on Kato et al.’s study. 11 Especially, its flow rate and mobile phase program were modified from the original method to be adapted to the column used in this study. All MS analyses of PFOA without derivatization were carried out in negative ion mode because the low pKa value of PFOA produced better MS sensitivity in negative ion mode than that in positive ion mode (data not shown) The only facile and selective fragmentation channel available for PFOA is the loss of CO2 and it was used as the SRM transitions both for PFOA and 13C8-PFOA analysis.12 The collision energy for SRM was optimized as 10 % of the full excitation voltage. Typical SRM chromatograms of PFOA and 13C8-PFOA obtained using the optimized LCMS/ MS condition are shown in Figure 2. The analytes were well-separated from the solvent front in the SRM chromatograms and showed sharp peaks appropriate for quantitative analysis. More notable chemical interferences for PFOA may be due to limited specificity of CO2 loss SRM transitions and more complex chemical noises in that particular m/z region.
>
The evaluation and optimization of SPE condition
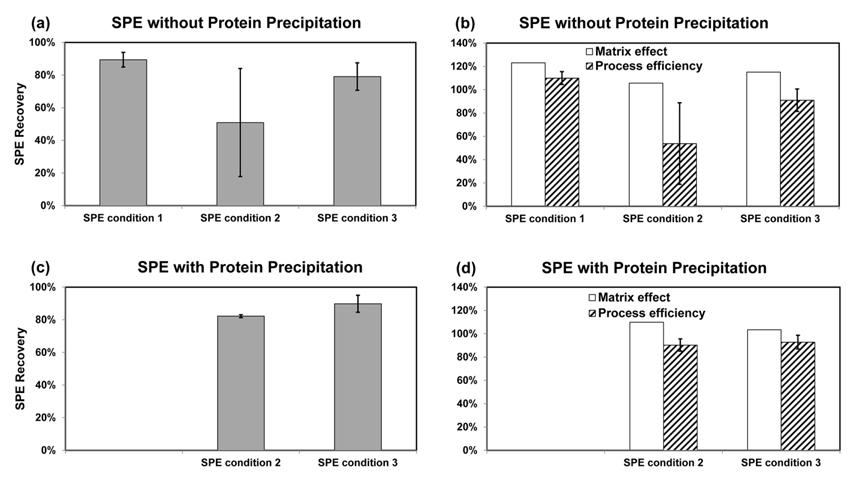
The recovery, matrix effect and process efficiency were evaluated from the ratio of test solutions B/C, C/A and B/A, respectively. The SPE condition 1 was a little modified from previous method and didn’t use protein precipitation.10 As shown in the Figure 3a, the recovery of the SPE condition 1 is about 90%. However, it shows matrix effect of signal enhancement. Without proper internal standard, it may result in positive bias in the measurement result of PFOA in serum. The SPE condition 2 and 3 without protein precipitation had lower recovery and poor reproducibility especially for the condition 2. Pre-treatment of FBS samples with protein precipitation, however, improved the recovery of SPE condition 2 and 3 significantly (Figure 3c). It also mitigated matrix effect as shown in the Figure 3d. Overall, prior protein precipitation treatment before SPE improved recovery of the analyte by SPE and minimized matrix effect. The SPE condition 3 with protein precipitation was chosen as the best optimized method with optimum recovery and matrix effect for PFOA in serum.
The HLB SPE and LC-MS/MS methods were optimized for the quantitative analysis of PFOA in serum. FBS spiked with the PFOA surrogate, 13C8-PFOA, before or after SPE was used as test samples for evaluation of the SPE efficiency. The best SPE efficiency with lowest matrix effect and maximum absolute recovery was obtained from SPE condition 3 combined with sample pre-treatment of protein precipitation. The SPE recovery of about 90% and negligible matrix effect were achieved for PFOA in FBS using the optimized analytical method. The present method will be used for the investigation of the clinical impact of PFOA in endocrine system related disease.