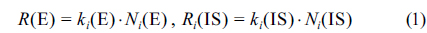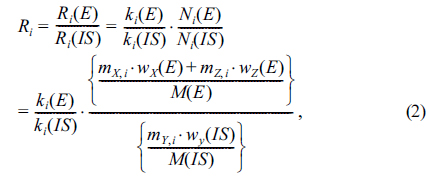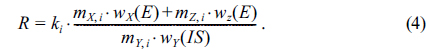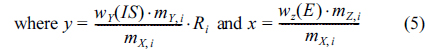Many countries set regulations on the concentrations of arsenic in drinking water and foods due to its well-known toxicity.1-4 Elevated dietary exposure to high levels of arsenic can be a potential threat to human health. Especially in sea weeds, arsenic has been frequently found in concentrations exceeding the regulation limits. In particular, laver has been traditionally consumed in large quantities in far-east Asian countries and, nowadays, they are gaining widespread popularity worldwide. Therefore, the accurate determination of arsenic in laver can be a matter of concern for the food safety in many counties.2-5 In order to assure the quality of measurement results, use of relevant certified reference material (CRM) has been recommended to analytical laboratories for the method validation and quality control in ISO/IEC 17025.6 The development of CRM for arsenic analysis in laver is also very important as a means to assist field laboratories to get reliable measurement results.
Accurate determination of elemental contents in a complex matrix is best realized using primary methods with the highest level of reliability including the isotope dilution (ID) inductively-coupled plasma mass spectrometry (ICP/MS). However, the ID ICP/MS analysis of arsenic is impossible because arsenic has only one stable isotope. Application of another potential primary method, the instrumental neutron activation analysis (INAA), is often not practically possible due to limited accessibility to facilities with neutron radiation source.7 As an alternative approach, development of an arsenic measurement method based on conventional analytical methods such as ICP/MS can be also considered provided that rigorous measurement and calibration strategies are implemented.
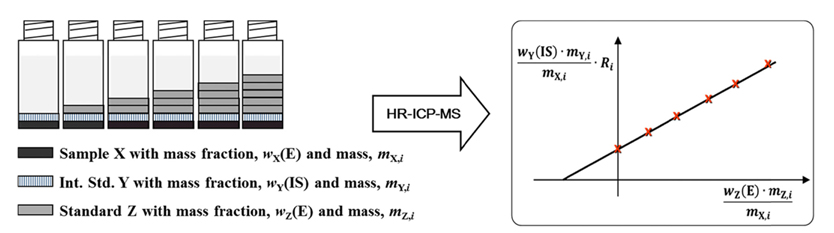
For the accurate determination of arsenic using ICP/MS, it is essential to exclude spectral interferences to 75As+ from isobars such as 40Ar35Cl+ and 59Co16O+. For this purpose, either higher mass resolution8 or collision/reaction cells are used. Non-spectral matrix effect is another important factor to be controlled to get right results from the ICP/MS measurement. Arsenic can be separated from the sample matrix to remove the matrix effect but incomplete recovery of arsenic and lack of robust recovery correction method poses a significant challenge to develop it as a reliable analysis method. Standard addition method (SAM) is one of the robust calibration method for ICP/MS analysis to overcome the measurement bias stemming from matrix effects without matrix separation.9,10 Provided that the procedure blank is tightly controlled and evaluated, it is expected to produce reliable result even in the presence of severe matrix effects. One of the pitfalls of the SAM, however, is relatively large uncertainty as a natural consequence of the extrapolation calibration. In order to achieve the highest level of accuracy to the extent of assigning the certified value for CRM development, gravimetric sample handling in the standard addition procedure is necessary as well as the use of appropriate internal standards.11 In this study, an ICP/MS-based arsenic measurement procedure was established with rigorous gravimetric standard addition method (SAM) combined with internal standard calibration as the most robust calibration strategy next to the ID ICP/MS. The method was applied to measure arsenic content in NMIJ 7402-a codfish CRM for method validation and certification of arsenic content laver candidate CRM.
The dry laver powder sample was provided by the National Institute of Metrology (NIM), China, which is a candidate reference material of the Asian Collaboration on Reference Materials (ACRM) program. The codfish CRM (NMIJ CRM 7402-a) used for method validation and quality control was obtained from the National Metrology Institute of Japan (NMIJ). The arsenic standard solution for standard additions and the germanium standard solution for internal standard were produced by Korea Research Institute of Standards and Science (KRISS) with traceability to the SI unit. The working standard solutions of the arsenic and the germanium were freshly prepared just before sample preparation by diluting the stock solutions of 1000 mg·kg˗1 with 5% (w/w) nitric acid. Nitric acids with various concentrations used throughout this study were prepared just before sample preparation by diluting the high purity concentrated nitric acid (≥65%) with deionized water (18MW·cm resistivity) from Milli-Q RG purification system (Millipore, USA). The high purity concentrated nitric acid was obtained by sub-boiling distillation of commercially available concentrated nitric acid from DONGWOO FINE-CHEM (Iksan, Korea). Sample preparation with
>
Sample preparation with standard addition procedure
About 0.2 g of laver powder from each bottle was sampled and weighed directly into a PTFE-TFM microwave vessel. Total six subsamples from different bottles of laver powder were prepared. Then, about 1 g of the germanium working standard solution with an appropriate mass fraction was added in each PTFE-TFM vessel and weighed exactly. For standard addition calibration, different amounts of the arsenic working standard solution were added in five levels from 1 g to 5 g, respectively, into the five PTFE-TFM vessels containing both sample and internal standard. The remaining one vessel was prepared without adding the arsenic working standard solution. Then, the samples in the six vessels were digested with 8 mL of concentrated nitric acid and 2 mL of 30% hydrogen peroxide solution using microwave digestion system (ETHOS PLUS, Milestone, USA). Microwave-assisted acid digestion was carried out with the temperature control program to increase temperature from room temperature to 200°C for 10 min and maintain it for 10 min. The digested samples were transferred into LDPE bottles and diluted appropriately for ICP/MS measurements. The analysis of the NMIJ 7402-a codfish CRM was also carried out for the validation of the developed method. Six aliquots (0.2 g each) of codfish powder from a single bottle were sampled individually in six PTFE-TFM microwave vessels. Then, the same procedure was used for preparation of standard addition samples.
For dry mass correction of laver powder, three subsamples were taken in parallel with the sampling for arsenic analysis. The subsample was about 0.2 g each and accurately weighed in a weighing bottle. After the samples in weighing bottles were dried at 102°C for 12 hours using a drying oven, the dried weights of the subsamples were measured. Finally, the losses of weights were used for the dry mass correction. In the dry mass correction for NMIJ 7402-a codfish sample, the procedure was the same except for taking 0.3 g of subsamples and drying at 102°C for 6 hours following the instruction of the certificate.
>
High resolution (HR) ICP/MS measurements of arsenic and germanium
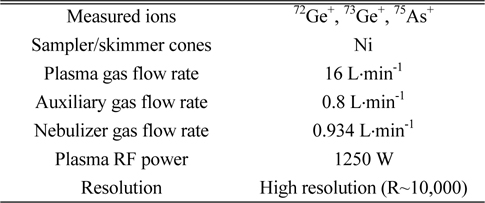
Signal intensities as counts per second (cps) of arsenic ions (75As+) and germanium ions (72Ge+ and 73Ge+) were measured using an HR ICP/MS (Element2, Thermo Fisher Scientific Inc., Germany) at high resolution mode. Sample solution was nebulized through quartz micro-concentric nebulizer at a flow rate of 400 mL·min˗1 into the Scott-type quartz spray chamber using a peristaltic pump. Fine aerosols generated in the spray chamber were introduced into the ICP plasma by quartz torch and injector. The operating conditions of HR ICP/MS are summarized in Table 1.
[Table 1.] Optimized operating conditions of HR ICP/MS for measurements of arsenic and germanium
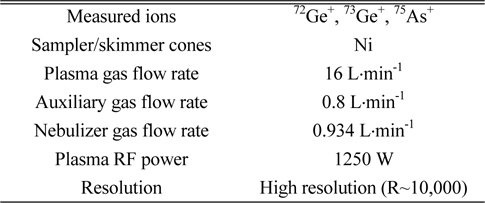
Optimized operating conditions of HR ICP/MS for measurements of arsenic and germanium
>
Gravimetric SAM with internal standard: Measurement Equation
The model equation for the gravimetric SAM for the analysis of the element, E, with the internal standard, IS, was derived for the analysis of total arsenic by ICP/MS. In this method, all the handlings of sample X, internal standard solution Y, and standard solution Z are carried out gravimetrically as shown by Figure 1. For an ith solution, mass
where
where
Then,
Finally, after rearrangement of equation (4), new linear equation can be obtained:
If the slop and y-intercept can be obtained from the linear plot for different standard addition solutions, the unknown mass fraction of sample,
>
Application of the gravimetric SAM: Determination of arsenic in codfish and laver samples
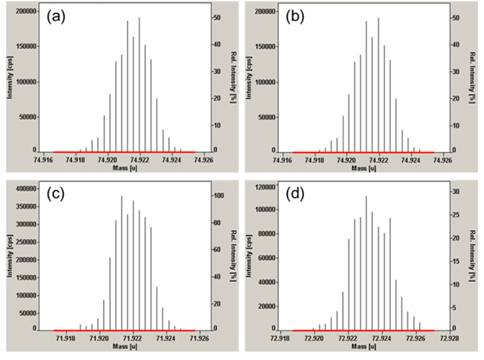
HR ICP/MS was optimized and applied to measure ion signals of arsenic and germanium. Figure 2(a) and 2(b) show typical mass spectra of 75As+ ions obtained from the sample and the arsenic standard solution, respectively. The resolutions were calculated more than 10,000 and no spectral interferences such as 59Co16O+ and 40Ar35Cl+ were observed even though 59Co16O+ ions can be shown on the right tail of 75As+ peak at this resolution. As the internal standard, ion intensities of two Ge isotopes, 72Ge and 73Ge, were monitored with the analyte isotope, 75As, by the fast electric-field scan. Very close mass-to-charge ratios of three isotopes allow fast electric field scanning at a fixed magnetic field with minimum mass dependent biases in the sector-field (SF) ICP/MS. Negligible natural level of Ge in the laver and codfish samples also made it an excellent choice as internal standard compared with Se, which is typically present in food samples. Among the five naturally occurring isotopes of Ge, 72Ge+ and 73Ge+ were chosen because of the absence and/or avoidable nature of isobaric interferences compared with the other isotopes of Ge. For example, 70Ge+and 74Ge+ interfere with 70Zn+ and 74Se+, respectively. Although the polyatomic interferences such as 56Fe16O+/32S40Ar+ and 57Fe16O+/33S40Ar+ also affect 72Ge+ and 73Ge+, respectively, their influences are easily removed by using high mass resolution in the SF ICP/MS.
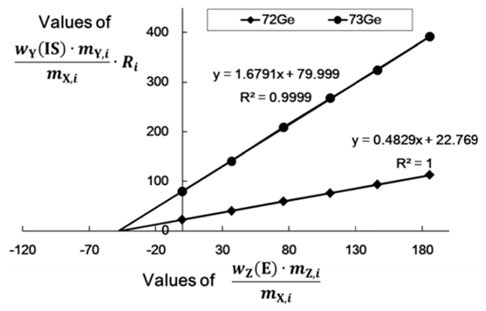
Standard addition calibration curves for determination of arsenic in laver samples using two different Ge isotopes, 72Ge and 73Ge, as internal standards are shown in Figure 3. From the calibration curve, mass fraction of arsenic was calculated. Both of the two calibration curves show excellent linearity with regression coefficient of 1.0000 and 0.9999, respectively, for 72Ge and 73Ge as internal standards. In the standard addition calibration, the ion signal ratios of 75As+/ 72Ge+ were in a narrow range of 0.5 to 1.4 (from 1.7 to 4.7 for 75As+/73Ge+) to make the linear regression sufficiently accurate approximation. It should be noted that the 6 data used for linear regression include variations due to between-bottle homogeneity. Although there was no noticeable difference between the two results obtained using the two different Ge isotopes as internal standards within the associated uncertainties, the result obtained using 72Ge as an internal standard was finally used for the analysis because of its smaller uncertainty. Coincidence of the two results also provides a supporting evidence on the robustness of the present method for arsenic determination. In the case of NMIJ 7402-a codfish CRM, which was used for method validation, total arsenic content was measured to be 37.07 mg·kg˗1 ± 0.45 mg·kg˗1 which is in good agreement with the certified value, 36.7 mg·kg˗1 ± 1.8 mg·kg˗1. The certified value of the total arsenic in the candidate laver CRM was determined to be 47.15 mg·kg˗1 ± 1.32 mg·kg˗1 (k = 2.8 for 95% confidence level) which is an excellent result obtained by SAM with only 2.8% of relative expanded uncertainty including the homogeneity between bottles. The uncertainties of determined value were calculated following the ISO Guide to the Expression of Uncertainty in Measurement (GUM) guidelines12.
A gravimetric standard addition method combined with internal standard and HR ICP/MS has been successfully developed for the accurate determination of total arsenic content in laver candidate CRM. A measurement model equation of gravimetric SAM with internal standard was derived. Standard addition calibration curves obtained for gravimetric SAM using Ge internal standard showed excellent linearity, which results in relatively small measurement uncertainties even though they include contributions from between-bottle homogeneity. The developed method was validated using NMIJ codfish CRM and applied for the certification of KRISS laver CRM. The present strategy is expected to provide an alternative means to certify elemental content in complex matrix, when the other primary methods of analysis, such as ID ICP/MS and INAA, are not available.