Enzyme immobilization is a promising method for the application of enzymes in industrial scale. The immobilized enzyme can be reused, and therefore, it could solve the problem of high cost of the enzyme. Silk fibroin has been used as a support of enzyme such as glucose oxidase (Asakura
Covalent bonding of enzyme on the surface of non-porous carrier can solve these problems especially the leakage can be prevented. Previously, we have covalently immobilized enzyme on the surface of degummed silk fiber (Lee
SS has high content of hydrophilic amino acids, such as serine, threonine, and aspartic acid, and thereby, much more hydrophilic compared to fibroin. In general, hydrophilic support is favored in enzyme immobilization (Secundo, 2013). Besides, for the immobilization of enzyme, a support must have sufficient binding sites in order to achieve high loading efficiency of enzyme. Compared to fibroin, SS has high content of lysine, arginine and histidine which provide reaction site during the covalent bonding of enzyme using glutaraldehyde (GA). These characteristics of sericin can be advantages for the enzyme immobilization because it can provide hydrophilic environment to the enzyme and allows high loading efficiency. In the present study, we have prepared pure sericin beads and adopted for enzyme immobilization.
Silkworm cocoons were kindly provided by Heung Jin Co. Ltd. All chemicals including trypsin, α-chymotrypsin, and lipase were purchased from Sigma-Aldrich (USA).
SS was extracted by boiling 25 g of Bombyx mori silkworm cocoons with 1 L of distilled water using an autoclave for 1 h at 120°C. The extracted solution was filtered with a nonwoven filter in order to remove the remaining cocoons. The SS solution was frozen at -70°C for 4 h and lyophilized.
>
Preparation and immobilization of enzyme on silk sericin beads
The lyophilized SS was dissolved in 1M LiCl/DMSO solution for 2 h at 50°C to prepare a dope solution of 20% (w/v). The dope solution was dropped into alcohol coagulants through a 18G syringe using a syringe pump (KD scientific, USA). Methanol and ethanol were used as coagulants, and the obtained beads are designated as SS-M and SS-E, respectively. The SS beads were left in the coagulant bath for another 1 h. They were then filtered with a nonwoven filter and washed with the same coagulant to remove the residual LiCl and DMSO.
In order to immobilize enzyme on the SS beads, the amine groups of SS were activated with GA. To ten beads of SS, 1 mL of 10% (v/v) GA in 0.2 M sodium carbonate buffer (pH 9.2) was added to activate the SS. The reaction was continued for 1 h at 25ºC. The activated SS beads were washed 2 times with distilled water and 3 times with 0.1 M sodium phosphate buffers (pH 7.4). An ice-cold buffer solution of enzyme (1 mg/mL) in 0.1 M sodium phosphate buffer (pH 7.4) was added to the activated SS beads and the reaction was carried out for overnight at 4ºC. The unbound enzyme was washed out 2 times with ice-cold 0.5 M of NaCl in 0.1 M sodium phosphate buffer (pH 7.4) and followed by 3 times with the same buffer but without NaCl.
>
Quantification and activity test of enzyme
The bound enzyme was measured by bicinchoninic acid assay methods using bovine serum albumin as standard. The amount of bound enzyme was calculated by subtracting the amount of remaining enzyme from the initial amount of enzyme. N-benzoyl-DL-tyrosine-p-nitroanilide hydrochloride (BTPNA) was used as substrate of α-chymotrypsin. The substrate solution were prepared by mixing 250 μL of 5 mM BTPNA in DMSO, 1 mL of distilled water and 150 μL of 0.1 M sodium phosphate buffer (pH 7.4). The substrate solution were added to the activated SS beads and incubated at 25ºC for 30 min. In order to measure the activity of the immobilized α-chymotrypsin, the increase of absorbance at 400 nm was measured using UV spectrometer (UVICON 923, Kontron Instruments, USA). If the value of absorbance exceeded 2, then it was diluted until it reached below 1.5. The activity of immobilized α-chymotrypsin was defined as μmol BTPNA hydrolyzed in 1 min applying an absorbance coefficient of ε400 = 9520 M-1cm-1. The stability against ethanol was measured by incubating enzyme immobilized SS beads in ethanol for 1 h at 25ºC. After that, the SS beads were washed 2 times with ice-cold 0.1 M sodium phosphate buffer pH 7.4, containing 0.5 M NaCl and 3 times with cold distilled water. The activity of immobilized enzyme after ethanol treatment was measured. For trypsin and lipase, 10 mM of N-benzoyl-DL-arginin-p-nitroanilid hydrochloride and p-nitrophenyl caprylate, respectively, was used instead of BTPNA and the pH of added buffer solution were 8. The increase of absorbance at 405 and 348 nm was measured for trypsin and lipase, respectively. The relative activity of immobilized trypsin and lipase were compared with each free enzyme (1 mg/mL).
Base on the previous study (Lee
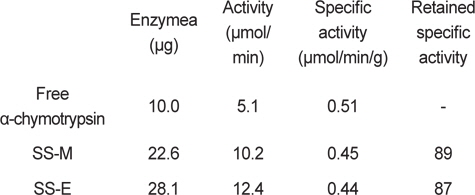
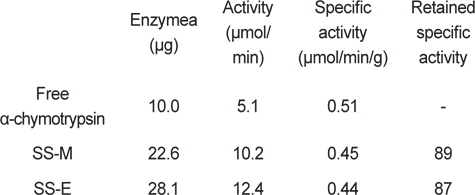
Amount of bound α-chymotrypsin and activity of immobilized α-chymotrypsin on silk sericin bead
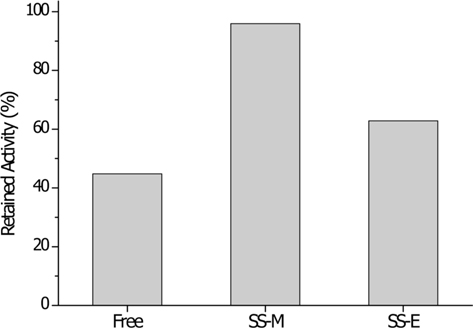
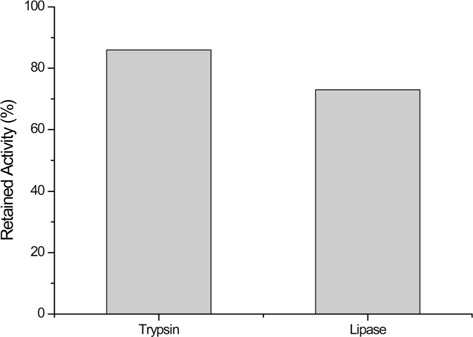
Trypsin and lipase were also immobilized onto SS-M bead. Compared to the free enzyme, trypsin and lipase retained their activity to 86 and 72%, respectively (Fig. 2). Unfortunately, the exact amount of enzymes bound to the SS-M bead cannot be calculated. However, the apparent activity shows that these immobilized enzymes have high preservation of activity after immobilization.
The hydrophilicity (or hydrophobicity) of a support is important in enzyme immobilization. Enzymes are proteins that consist of hydrophilic and hydrophobic part. When enzymes are immobilized on a hydrophobic support, then water molecules would be expelled from the support, which result in less water molecules around the immobilized enzyme. Therefore, there is an increased hydrophobic interaction between the support and the hydrophobic core of enzyme. This induces structural transition of enzyme, and finally, the enzyme will lose the activity by denaturation. The opposite effect is expected when hydrophilic support is used. It will retain more water molecules at the surface of support and establishes friendlier environment to the enzyme. The good preservation of enzyme activity upon immobilization on SS bead might be explained in this manner.
Although more detailed study is required to prove the effectiveness of SS bead in enzyme immobilization, the current preliminary result shows that SS could be a good candidate material for enzyme immobilization support. SS has been considered as useful biomaterial for long time (Zhang, 2002), but up to now, it has been shown very little application. Although application of SS in biomedical fields has been proposed (Aramwit