Silk fibroin (SF) is a biocompatible material with various biomedical applications, for instance as a drug delivery vehicle or a scaffold in tissue engineering (Ki
SF is initially prepared as an aqueous solution before being manufactured into various forms including fiber, film, sponges, nano- and microparticles, and hydrogel. The multi-step process includes the removal of sericin by degumming using sodium carbonate solution with or without surfactant. The SF is then dissolved in 9.3 M LiBr solution, which is accelerated by higher temperatures: complete dissolution requires more than 12 h at room temperature, but at 60℃ or higher the time is reduced to less than an hour. The removal of excess LiBr by dialysis is the rate-limiting step during the preparation of SF solution, with a recommended time of more than 48 h (Rockwood et al., 2011).
Despite being a time-consuming process, dialysis is the only applicable method for complete LiBr removal. Other techniques, such as membrane separation or electrodialysis, each have their limitations. Although LiBr can be extracted using a membrane within a few hours, membrane fouling by SF necessitates high pressures to maintain adequate flux, which induces SF crystallization and leads to the formation of insoluble fleeces. Electrodialysis is also a well-established technique for removing salt from a solution, but in the case of LiBr, hazardous Br2 gas is generated at the anode. LiBr is harmful if swallowed and causes skin and eye irritation; thus, the removal of LiBr is important for biomedical applications of SF. However, there is little information available regarding the effects of residual LiBr on the structure or cytotoxicity of SF-based materials; these were investigated in the present study using SF films with variable amounts of LiBr prepared by varying the dialysis time. The secondary structure of SF films was determined by Fourier transform infrared (FT-IR) analysis, while cytotoxicity was assessed by treating cultured cells with SF film extract or by growing cells directly on SF film and evaluating their viability using the MTT assay.
Silk cocoons were obtained from the National Academy of Agricultural Sciences (Seoul, Korea). Sodium oleate was purchased from Junsei Chemical Co., Ltd. (Tokyo, Japan), and LiBr was from Thermo Scientific (Waltham, MA, USA). All other chemicals were purchased from Sigma-Aldrich (Seoul, Korea).
>
Preparation of SF aqueous solution
Cocoons were cut into 8 equal-sized pieces, which were immersed in degumming solution consisting of 0.3 wt% sodium oleate and 0.2 wt% sodium carbonate. Degumming was performed at 100℃ for 1 h at a final rate of 28.1%. SF fibers were dried in an oven at 50℃ for 2 d, and 40 g were dissolved in 200 mL 9.3 M LiBr solution at 50℃ for 4 h.
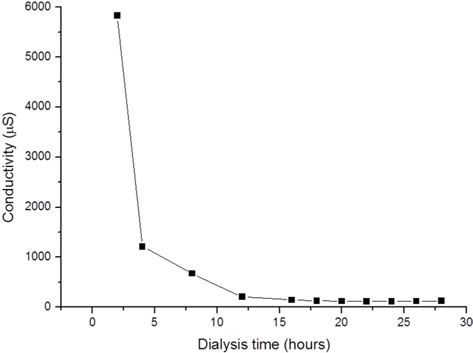
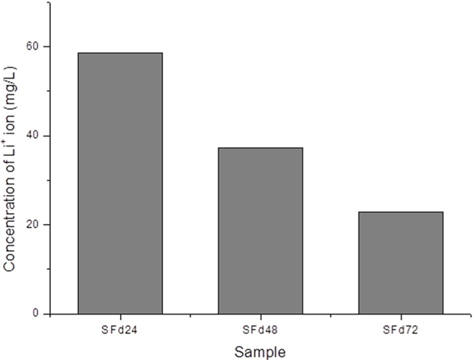
After dissolving the SF fibers, the solution was dialyzed using a dialysis tube (Spectrum Laboratories, Inc., Rancho Dominguez, CA, USA) with a diameter and length of 25.5 and 300 mm, respectively, and a molecular weight cut-off (MWCO) of 6–8 kDa; each tube containing 50 mL solution was hermetically sealed and immersed in 5 L distilled water. The dialysate was changed every 2 h five times, then every 4 h until the end of the dialysis. To obtain solutions with different amounts of residual LiBr, the dialysis was terminated at 8, 24, 48, and 72 h. The conductivity of the dialysate during the procedure was measured using a conductivity meter (Model 115; ATI Orion, Boston, MA, USA). The amount of residual lithium ion in the dialyzed SF solution was measured with an inductively coupled plasma optical emission spectrometer (ICP-OES) (ICP-730-ES; Varian, Inc., Belrose, Australia).
>
Preparation and characterization of SF films
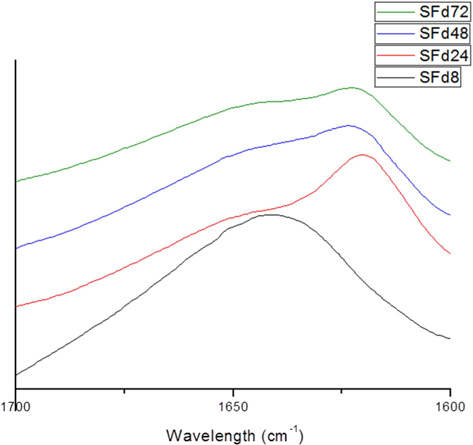
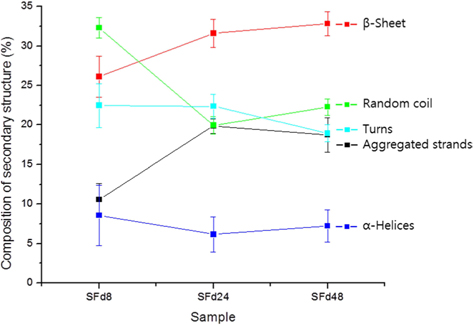
To obtain SF films from solutions corresponding to different dialysis times—which were designated as SFd8, SFd24, SFd48, and SFd72—Petri dishes were coated with solution and dried at 50℃ for 24 h. With the exception of SFd8, the films were insoluble after casting. The secondary structure of the films was determined using an attenuated total reflectance (ATR) FT-IR spectrometer (ATR-FTIR, Nicolet 6700; Thermo Scientific) as in previous studies, while composition was determined at the amide I band between 1600 and 1700 cm-1 using the peakfitting method. The second-derivative spectra for calculating the number and position of overlapped single bands and the deconvolution spectra for fitting were obtained using Origin 8.0 software (Northampton, MA, USA). Curve-fitting proofs were performed until the χ2 distribution reached the minimum value with multi-peak Gaussian fitting. The relative area of the overlapped single bands was used to estimate the proportion of each type of secondary structure, and the assignment of single bands was as described in previous studies: 1605–1615 cm-1, aggregated strands; 1616–1637 cm-1, β-sheet; 1638–1655 cm-1, random coil; 1656–1662 cm-1, α-helices; and 1663–1695 cm-1, turns (Zhang
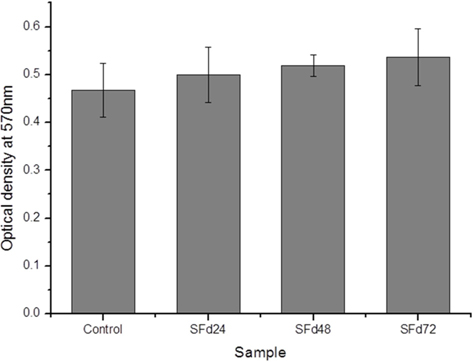
The cytotoxicity of the SF film was evaluated directly and indirectly using the MTT cell viability assay. For the indirect assessment, cultured NIH3T3 fibroblasts (SPL Life Sciences Co., Ltd., Pocheon, Korea) were treated with SF film extracts, obtained by immersing SF film prepared from solutions with different dialysis times in serum-free medium for 24 h and collecting the medium. Cells were seeded in a 24-well plate (SPL Life Sciences Co., Ltd.) at a density of 1.0 × 104 cells/well and incubated at 37℃ and 5% CO2/95% air for 24 h to ensure complete attachment. The culture medium was then replaced with extract-containing medium and incubated for 24 h before cytotoxicity was assessed using a standard MTT assay: 200 μL of 0.5 mg/mL MTT was added to each sample, and after 4 h, the supernatant containing formazan crystals produced from the MTT substrate by mitochondrial activity was removed and solubilized in 200 μL dimethylsulfoxide (DMSO; Sigma-Aldrich, St. Louis, MO, USA). UV absorbance was read at 570 nm using a microplate reader (Synergy H1; BioTek, Winooski, VT, USA).
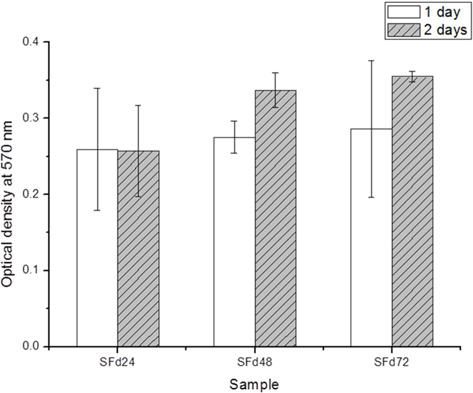
For the direct contact test, cells were cultured on SF film sterilized by immersion in 70% ethanol for 30 min followed by three 20-min phosphate-buffered saline (PBS) washes. The film was transferred to 24-well plates, and cells were seeded at a density of 1.0 × 104 cells/well and maintained at 37℃ and 5% CO2. Cell culture was performed at 37℃ and 5% CO2. After the incubation period, 200 μl of 0.5 mg/mL MTT was added to each sample for 24 h. The MTT solution was removed, and samples were washed twice with Dulbecco’s PBS (DPBS), and 200 μL DMSO was added to solubilize the formazan crystals. After 30 min, UV absorbance at 570 nm was measured. Each experiment was performed in triplicate and data are shown as the mean ± standard error of three measurements.
>
LiBr concentration during dialysis
Dialysis removes excess LiBr used to dissolve SF, and requires several dialysate changes; according to one report, at least 6 changes within 48 h are needed (Rockwood
>
Effect of residual LiBr on the secondary structure of SF films
The original ATR-FTIR spectra of SF films and secondary structure composition after deconvolution are shown in Fig. 3 and 4, respectively. Only SFd8 film was still soluble after casting; the insolubility of the other films was likely caused by a transition in secondary structure. Indeed, the secondary structure of the SFd8 film consisted mostly of random coils, whereas β-sheets were more prominent in SFd24, SFd48, and SFd72. The deconvolution could not be carried out on SFd72 due to low intensity in the range of 1700–1680 cm-1. However, the peak intensity at 1620 cm-1 was higher for SFd72 than SFd24 and SFd48, indicating a higher degree of β-sheet formation. These results suggest that a high residual lithium ion content hinders conformational changes, as in the case of SFd8. Conversely, the removal of lithium allows the structural transition of SF to a β-sheet structure. The role of various cations on structural transitions has been previously studied; for instance, it has been reported that divalent calcium ions can induce β-sheet formation by bringing SF molecules closer together and acting as an ion bridge (Foo
Effect of residual LiBr on the cytotoxicity of SF films
Residual LiBr was detected in SF films even after extensive dialysis (Fig. 2). LiBr has acute oral toxicity with an LD50 of 1800 mg/kg, which is low enough to exclude it from categorization as a toxic substance. Nonetheless, any biomaterials require rigorous testing in order to ensure that they are safe for use. In particular, SF has low biodegradability, and would therefore persist at the site of implantation over a long period. Even at a low level of toxicity, prolonged exposure can be harmful. The cytotoxicity of the SF film was verified indirectly by using SF film extract to treat cultured cells, and directly by seeding cells onto the film. When dialysis was performed for more than 48 h, the cytotoxicity of the SF film was negligible (Figs. 5 and 6). The results of the MTT assay revealed that the soluble SF extract was not toxic to cells for any dialysis time (Fig. 5). However, at least 48 h of dialysis were required to counter any adverse effects on cell viability, which was unaffected by SFd48 and SFd72, but was significantly reduced by SFd24 (Fig. 6). Lithium reportedly inhibits the glycogen synthase kinase-3β, a serine/threonine kinase that controls cell cycle progression; lithium treatment causes G2/M arrest while preserving cell viability (Mao