Pebrine is a deadliest disease of mulberry silkworm,
Therefore, role of transovum transmission of pebrine spore through accessory sex organs / external body surface of male moths in successive generations for transmission of disease was undertaken to find out the sole mechanism of outbreak of disease.
>
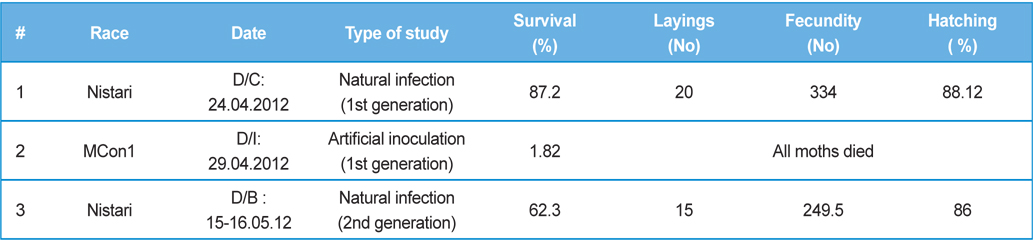
Rearing of 1st generation of infected silkworm
One hundred live cocoons were collected from one farmer’s house at Barunighata village, Birbhum district, West Bengal, India. Fifty cocoons were subjected for isolation of
>
Rearing 2nd, 3rd and 4th generations of infected silkworm
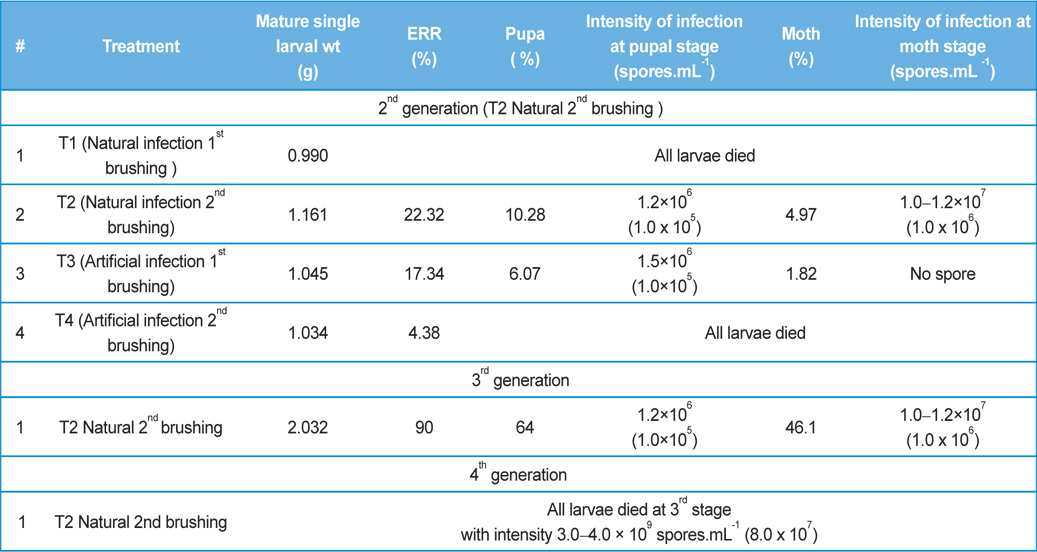
Rearing of 2nd generation were conducted with fifty larvae (Race: Nistari) of 1st brushing (T1) and fifty larvae of 2nd brushing (T2) when eggs were prepared utilizing moths recovered from natural infection of 1st generation. Rearings of 2nd generation were also conducted with fifty larvae of 1st brushing (T3) and fifty larvae (Race: Nistari) of 2nd brushing (T4) when eggs were prepared utilizing moths recovered from artificial infection of 1st generation.
Rearing of 3rd generation was conducted with 500 larvae when eggs were prepared utilizing moths recovered from natural infection of 2nd generation (T2).
Rearing of 4th generation was conducted with 1000 larvae when eggs were prepared utilizing moths recovered from natural infection of 3rd generation (T2).
>
Rearing 2nd, 3rd and 4th generations of infected silkworm with protection
All the procedure was same as Rearing 2nd, 3rd and 4th generations. However, these experiments were conducted in two separate batches, one with protection using existing disease management systems, using ‘Labex’ as bed disinfectant and ‘5% Bleaching powder’ as room disinfectant and other with new disease management system using ‘Sericillin’ as bed disinfectant and ‘fumigant chemicals’ as room disinfectant.
>
Rearing 3rd generations of infected male with healthy female
Here, we have crossed the fully infected male moths (1.0-1.2 × 107 spores.mL-1) recovered from rearing 3rd generations with healthy female moths. We have continued the generations with recording survival percentage and intensity of infection.
In rearing of 1st generation of infected silkworm (
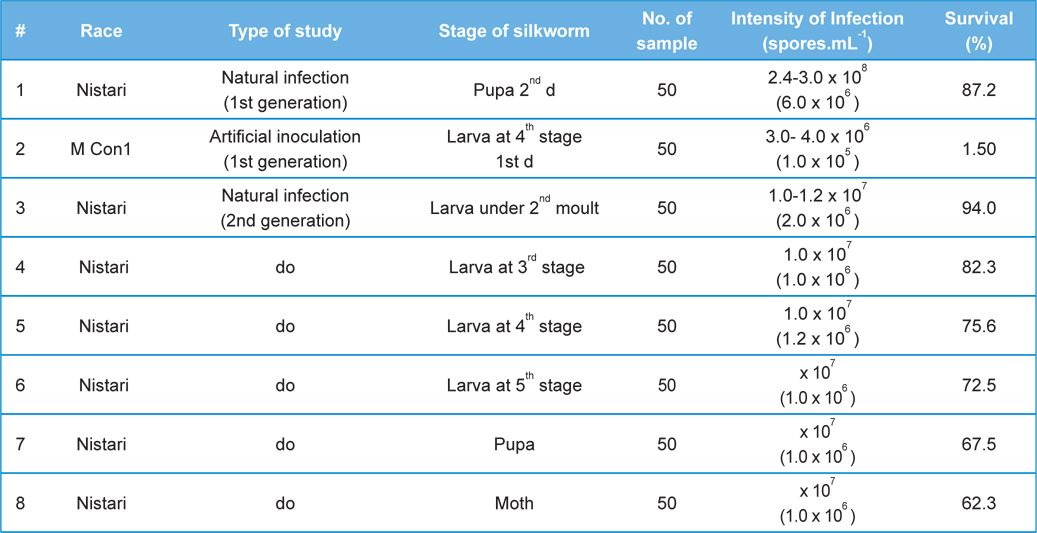
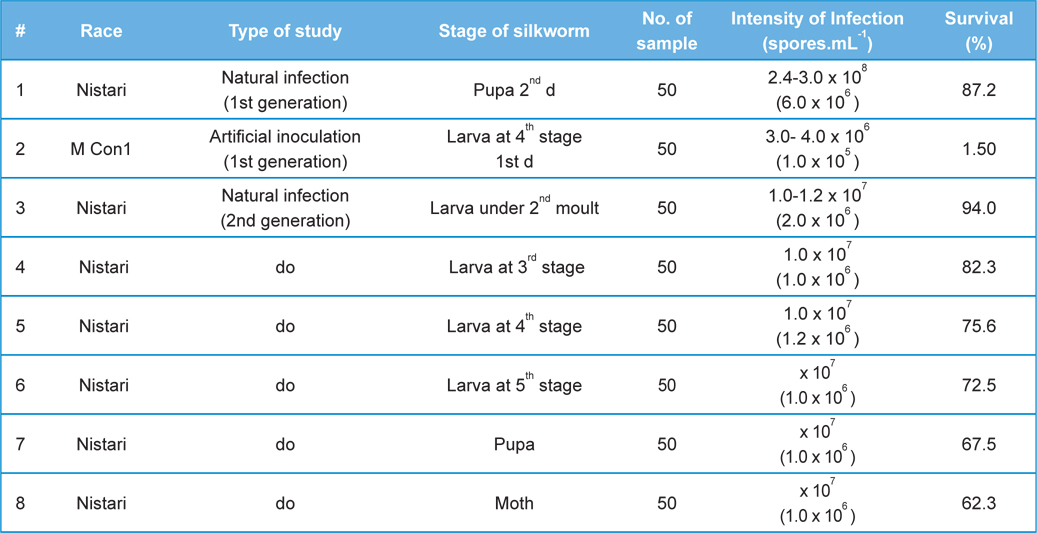
Comparative performances of N.bombycis at different metamorphic stage of B. mori L. during artificial and natural infection (data in parenthesis indicates the standard error of mean)
[Table 2.] Fitness Efficiency Performance of B. mori L. during artificial and natural infection
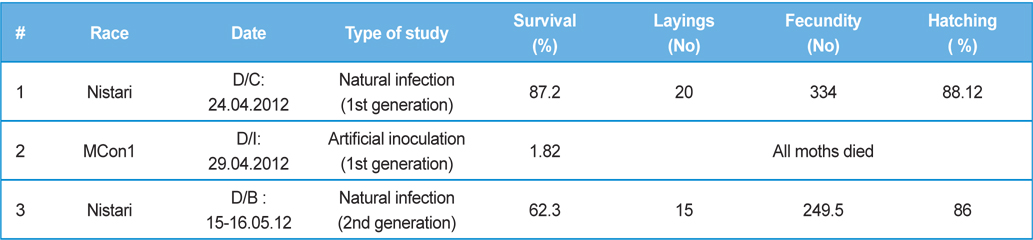
Fitness Efficiency Performance of B. mori L. during artificial and natural infection
In rearing of 2nd, 3rd and 4th generation of infected silkworm (
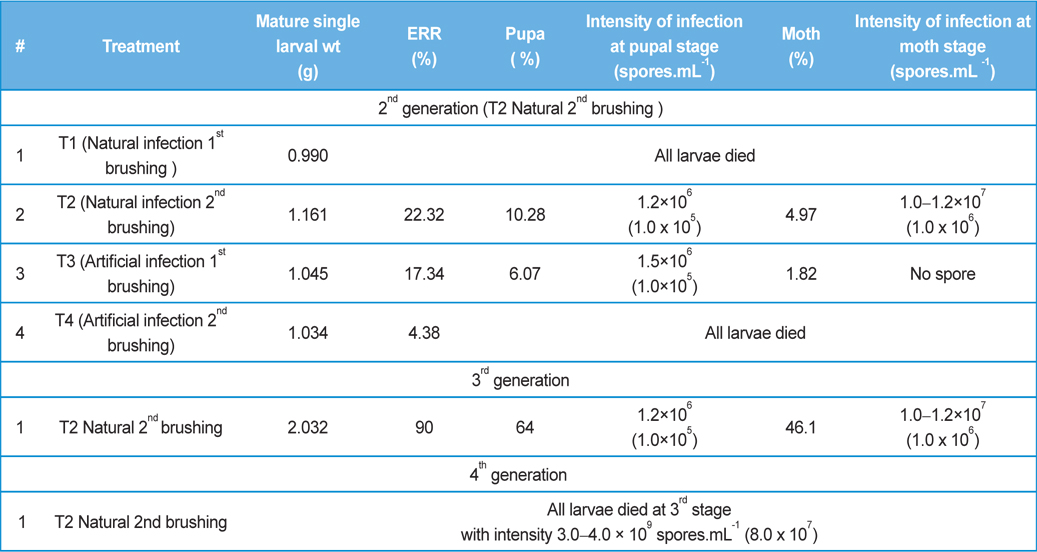
Comparative performances at different metamorphic stage of B. mori L. during different brushing practice at artificial and natural infection (data in parenthesis indicates the standard error of mean)
In rearing of 2nd, 3rd and 4th generation of infected silkworm with protection (
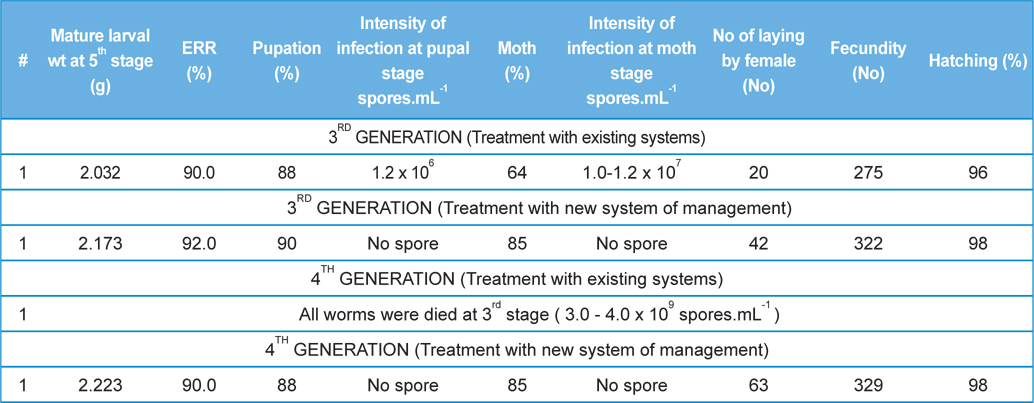
Comparative performances of management for control of pebrine disease of B. mori L. using existing system of management and new integrated system of management.
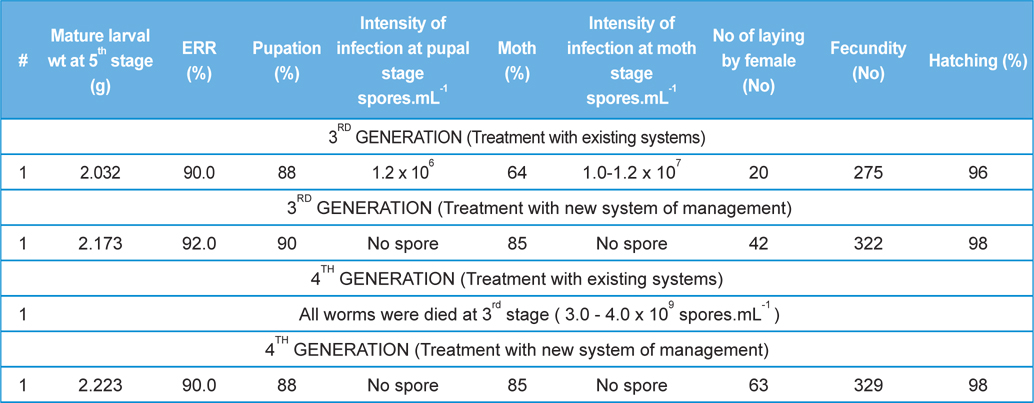
In rearing of 3rd generation of infected male with healthy male (
[Table 5.] Secondary contamination of pebrine disease in successive generations in B.mori.
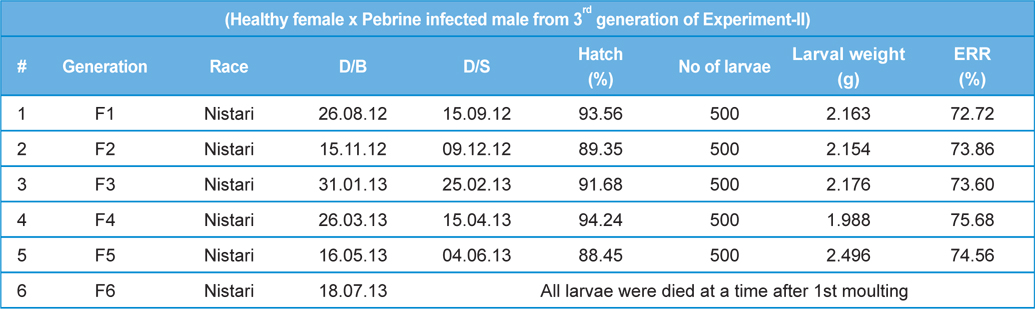
Secondary contamination of pebrine disease in successive generations in B.mori.
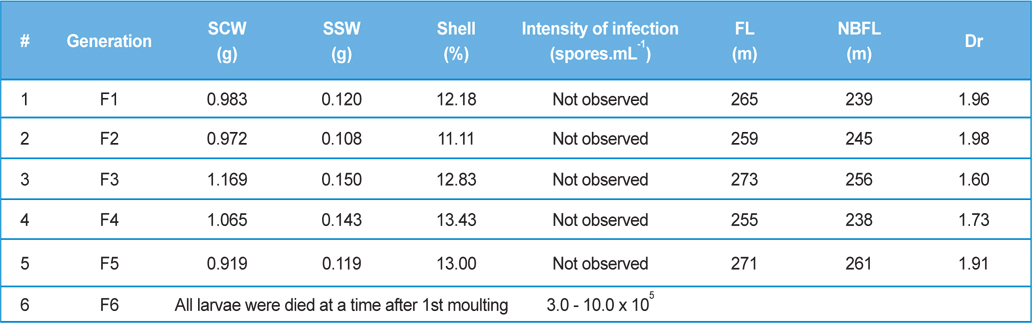
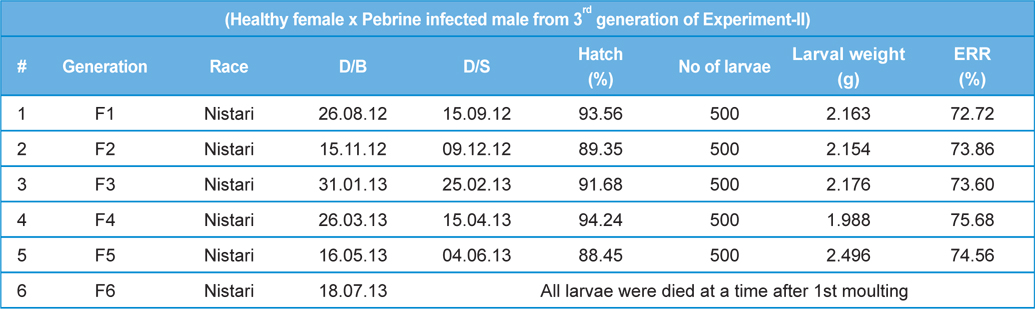
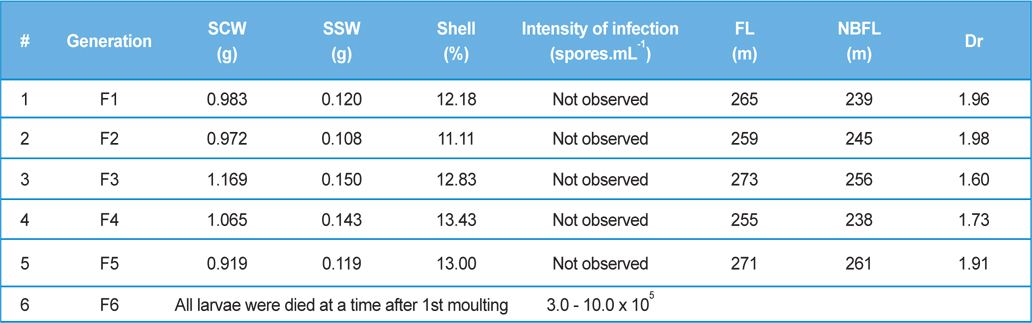
Intensity of infection and reeling performance of secondary contamination of pebrine disease in successive generations in B.mori. (Healthy female x Pebrine infected male from 3rd generation of Experiment-II)
Time taken for establishment of the pathogen for completion of its life cycle and production of spores depends on inoculum load and other environmental factors (Steinhaus and Huges, 1949). We have observed in our previous study that spore harvest was more in male moth compared to that in female moth though the inoculum concentration, source of pathogen and the rearing were conducted in the same environment (Chakrabarty
From the study, it may be concluded that both male and female moths to be examined microscopically during hybrid laying preparation. We could observe only 4th generation from parental generation (P3) to commercial rearing in the field. As there is no chance for 6th generation study in the field, for that reason outbreak of pebrine disease from secondary transmission is not observed. However, we should take care where parental generation (P3, P2 and P1) is maintained to check the secondary transmission. Besides, new management system using ‘Sericillin’ as bed disinfectant with ‘fumigant chemicals’ as room disinfectant is required to be adopted, especially where parental generation is maintained i.e., seed production centre, to control the secondary transmission of pebrine disease.