Rapid industrialization, environmental pollution, westernized diets, and increased stress due to complex social structures have resulted in the advent of a number of new diseases. Diseases such as diabetes, hypertension, obesity, and atopic dermatitis, whose incidence was lower in the past, occur more frequently today. In particular, the incidence of atopic dermatitis has dramatically increased because of rapid increases in the number of allergens that induce various immune hypersensitive diseases; this increase in allergens is due to the excessive utilization of artificial chemicals and the acceleration of environmental pollution. In recent years, the number of child patients with atopic dermatitis has notably increased in Korea; according to the National Health and Nutrition Examination Survey in 2008, 19.2% of 1–5-y-old children (1 in 5 children, or 0.5–1% of the entire population) suffers from atopic dermatitis (Nam
Atopic dermatitis (AD) is a chronic inflammatory dermatitis that presents with itchiness and inflammation. When allergens (the causative factors of the allergy) enter the body, IgE is produced in B-cells (in the blood) and transported to mast cells in the skin. It then combines with basophils (a type of white blood cell), and histamine is formed and stored; upon repeated exposure to the same allergens, allergic symptoms occur (Harifin and Rajka, 1980; Horsmanheimo
Because atopic constitution is essentially incurable, the management of AD should focus on the avoidance of causative factors, and control through appropriate treatments, instead of complete recovery. As of now, chemical drugs such as steroids (adrenocortical hormones), antihistamines, and antibiotics are widely utilized as treatments for AD. Steroids are very effective in anti-inflammatory actions and immune suppression but their long-term utilization may show side effects such as skin weakening, systemic hormone symptoms, and addiction. Antihistamines prevent histamine induction in mast cells thereby reducing itchiness, but are used as temporary treatments; their long-term administration can result in side effects such as insomnia, anxiety, and loss of appetite (Koblenzer, 1999; Mihara
Silkworm feces are nontoxic, prevent paralysis of the body and limbs, and strengthen organs. They are also effective in the treatment of skin diseases and possess anti-inflammatory and analgesic properties. They are also effective for the treatment of diabetes and are rich in various nutritional components (Kim
However, the value of utilizing silkworm feces that are obtained from
This study evaluated the anti-atopic effects of silkworm feces extracts in DNCB-induced NC/Nga atopic mice in order to obtain basic data for the development of safe and effective atopy treatment compositions or functional cosmetics without side effects.
>
Experimental animals and samples
Eight-week-old NC/Nga mice were utilized in this study. Seven-week-old mice were purchased from Central Laboratory Animals Co., and food and water were given ad libitum during the study period. They were acclimated in the room at 22℃ and a humidity of 40–50% with an automatically controlled dark cycle (12 h) for one week. Healthy mice with constant weight were selected for this study. The NC/Nga mice utilized in this study showed AD concomitant with overproduction of IgE that displayed clinical and histological similarities with lesions of human AD; the mice were extremely sensitive to hypersensitivity reactions induced by X-ray radiation and ovalbumin, and thereby developed eczematous dermatitis in a generic environment (Norikazu
Hypersensitivity inducers for the purpose of facilitating AD, 2,4-dinitrochlorobenzene (DNCB; Sigma Co., USA), were dissolved in solvent (AOO solution, acetone/olive oil = 3:1, v/v) at concentrations of 1.0% and 0.2%. Silkworm feces (500 g) were placed in a 3 L conical flask and 80% EtOH solution (2.5 L) was added followed by extraction for 10 h using ultrasonic waves. The extracts were filtered and the remaining silkworm feces were placed in a 3 L conical flask again. Then, they were extracted by 80% EtOH (2.5 L) and filtered. Primary extracts and secondary extracts were combined, and the combined extracts were subjected to evaporation under reduced pressure using a rotary evaporator at <40℃ followed by freeze drying. They were stored at -70℃ until further analysis (recovery rate 17.5%).
>
Atopic dermatitis induction and silkworm feces extracts treatment in NC/Nga mice
Hair from the back of the mice was removed entirely and the mice were left for 24 h to cure micro-damages in the skin that might have occurred during hair removal. After 24 h, 1% DNCB (100 μL) was spread on the back of the mice for four days in a row (except for the Normal group) in order to induce AD. The DNCB spread was stopped for three days and 0.2% DNCB (100 μL) was treated twice a week and acute AD was maintained for a total of 11 wk. Twelve weeks after the DNCB spread, silkworm feces extracts suspended in deionized water were prepared at the concentration of 1 mg/mL, 3 mg/mL, and 10 mg/mL, and a 200 μL treatment was applied once a day five days in a row for four weeks. Skin pigmentation from silkworm feces and skin rash were visibly measured. Serum was collected and clinical changes were observed.
>
Tissue stain and observation
Skin tissues were iNCised as 1.5 ×1.5 cm squares based on peripheral affected area and fixed with 10% formaldehyde solution followed by storage at 4℃ until tissue staining. For this, hematoxylin-eosin (H&E) stain was employed. In the H&E stain, the slide with skin tissues attached was first treated with 100% methanol for 1–3 min; OCT compound was melted, and the tissues were fixed. They were then washed with water for 1 min and stained in hematoxylin solution for about 3 min. After washing with distilled water, they were treated with 70% ethanol for four times and washed with distilled water followed by performing the same protocol as the previous steps in 0.2% ammonia solution. They were then treated with eosin for 30 s and the cytoplasm was stained and dried. Permount mounting medium was added on top of the tissues and covered with cover slips. Overall skin conditions, inflammatory cell shift, and epidermis thickness were observed under a 100X optical microscope.
>
Atopic dermatitis treatment efficacy evaluation indicators
Histopathological indicator analysis methods were utilized in order to evaluate efficacy of AD treatment. Indicators of dermatitis were used on the basis of epidermis necrosis, epidermis thickness, and infiltration of inflammatory cells in dermis. The indicators were shown as a sum of the divided outcomes that were observed under a 100X optical microscope. Ten sections per mice were observed and averaged.
PreseNCe of necrosis and its degree in the entire epidermis were semi-quantified by assigning a severity score as follows: 0 when there was no necrosis; 1+ when there was necrosis in 1/3 of the top part of epidermis based on the worst part; 2+ when there was necrosis in 2/3; and 3+ when there was complete necrosis.
Thickness was measured in 4-5 parts of the entire epidermis evenly and was averaged.
3) Infiltration of inflammatory cells in dermis
Scores were given as follows: 0 when inflammatory cells were absent in dermis; 1+ when inflammatory cells were partially localized or were multiple but rarely observed; 2+ when inflammatory cells were multiple and commonly observed; 3+ when there were severe infiltration of inflammatory cells.
>
Immunoglobulin E (IgE) concentration measurement
Blood (0.5 mL) was collected from the hearts of the mice using tube-type syringes treated with EDTA. The collected blood was centrifuged at 3000 rpm and 4℃ for 10 min to separate serum. The serum was stored in a deep freezer until further analysis and serum IgE concentration was determined using a Mice IgE ELISA kit (Shibayagi Co., Ltd, Japan). Then, 50 μL of a mixture of 5 μL of serum collected from the mice and 45 μL of dilution buffer was placed in each well of a 96-well plate and incubated on a shaker at room temperature for 2 h followed by washing three times with washing buffer solution. Biotinconjugated anti-IgE was then added and incubated at room temperature for 2 h followed by washing three times. HRP-conjugated avidin (50 μL) was added and subjected to incubation on the shaker at room temperature for 1 h and washed again. Chromogenic substrate (TMB) reagent (50 μL) was placed and incubated for 5 min. Then, 50 μL of stop solution was added and absorption was measured via ELISA reader (450 nm).
>
Cytokine coNCentration measurement and expression change analysis
Using serum samples obtained by centrifugation of the mice blood, the concentrations of blood TNF-α and IL-4 were measured using an enzyme immunometric assay kit (Assay Designs, INC. USA). Capture antibody (100 μL) that was 180 times diluted with PBS was added to a 96-well plate and incubated overnight at room temperature. Then, block buffer (300 μL) was added and reacted at room temperature for at least 1 h. Next, 100 μL of each sample diluted with reagent diluents and standard solution was placed and reacted at room temperature for 2 h. Streptavidin-HRP was diluted with working dilution and 100 μL of the solution was added to each well and reacted at room temperature for 20 min. Substrate solution (100 μL) was then added and reacted at room temperature for 20 min again. Each step was washed with 0.05% PBS-Tween. Lastly, after color development, 2N H2SO4 (50 μL) was added in order to stop the reaction followed by measurement via ELISA reader (450 nm). Moreover, to analyze expression changes, total mRNA of blood TNF-α, IL-1β, and IL-4 was separated and mRNA expression was measured by electrophoresis.
The statistical significance was tested via the student’s t test and the histological severity score was tested using ANOVA(one way analysis of variance). A p-value less than 0.05 was considered as statistically significant.
>
Atopic dermatitis induction when treating DNCB in NC/Nga mice
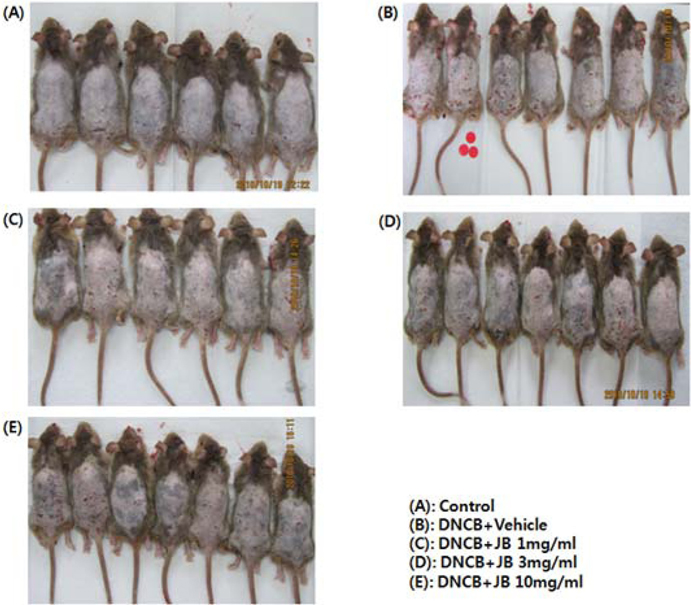
The effects of silkworm feces extracts determined via visual comparison of the mice are shown in Fig. 1. After the hair of the mice was removed, spreading of the DNCB solution formed additional dermis; and various intense AD symptoms such as skin spots, red spots, skin dryness, edema, and bleeding were induced. Itchiness, the most common symptom of skin diseases, can be caused by inflammation, cancer, metabolic diseases, infection, mental diseases, drug administration, and stress; and the activation of mast cells aggravates AD in humans (Stander
>
Histopathological evaluation
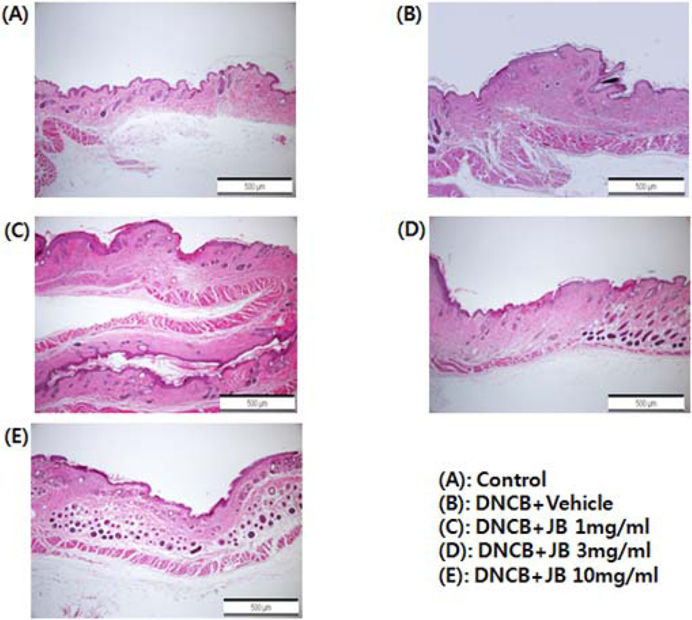
Results of the histopathological evaluation after with silkworm feces extract treatment (1 mg/mL, 3 mg/mL, and 10 mg/mL) in DNCB-induced AD mice are shown in Fig. 2. During the tissue specimen test, silkworm feces extract treatment in the DNCB-induced AD treatment group (Fig. 2A) resulted in the formation of uniform and smooth dermis layers with a surface thickness of the epidermis layer similar to that in the normal group, and the formation of connective tissues in a more constant direction, confirming that the skin was similar to that in the normal group. Further, regeneration of skin epithelial tissues, formation of dead skin cell layers, proliferation from basal layers to reticular layers, and formation of accessory organs were clearly activated, and vascular tissues were observed to be thickly, densely, and evenly formed in a constant direction (Fig. 2C-E). The surface of the skin in the AD mice group was extremely tough due to numerous dead skin cells and showed local edema; a notable loss of basal layer cells was found in the epidermis, and cells associated with inflammation (lymphocytes, mast cells, and eosinophils) were observed in the epidermis and dermis (Fig. 2B). In the silkworm feces treatment group, on the other hand, such cells were hardly seen and the thickness of the epidermis layer was found to gradually recover and become similar to that of the normal control group. In the skin tissues of individuals with allergic contact dermatitis, degranulated mast cells and eosinophils infiltrate the skin tissues, and excess keratinization of epidermis occurs (Vestergaard
>
Pathologic evaluation outcomes of each indicator
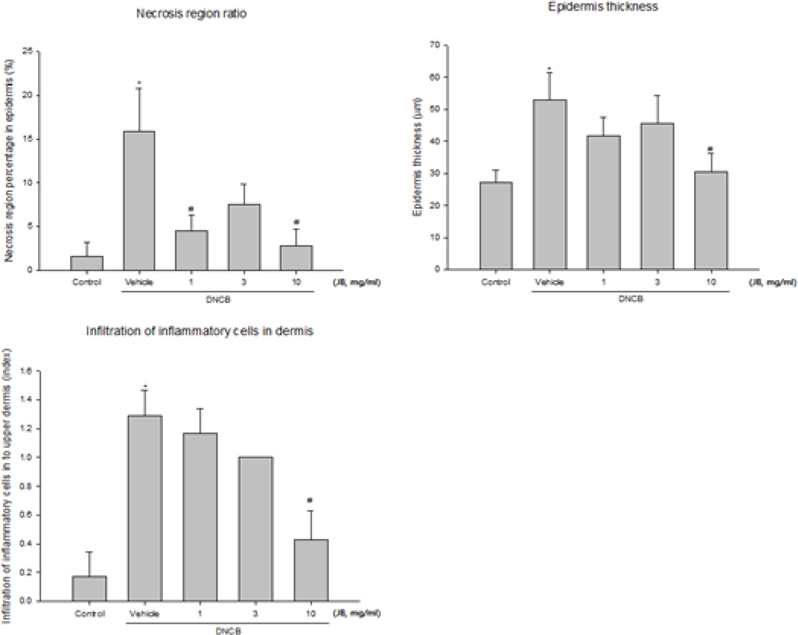
Proportions of the epidermis necrosis area in the AD induced area are shown in Fig. 3A. Only partial epidermis necrosis was observed in the silkworm feces extracts treatment groups but the groups treated with 1 mg/mL and 10 mg/mL silkworm feces showed a significant decrease. Epidermis thickness (µm) was measured in 4-5 places throughout entire epidermis and was averaged; statistical significance was tested with respect to the concentration of silkworm feces extracts treatment (Fig. 3B). Epidermis thickness was found to be decreased in all silk feces extracts treatment groups compared to the DNCB-treated mice and significant changes were observed in the 10 mg/mL treatment group, thus confirming the correlation between atopy and skin thickness. Likewise, due to increased percutaneous moisture loss, percutaneous dead skin cell layers such as scab, scales, and lichenification occurred in the atopy-induced skin thereby increasing skin thickness; but after the experimental treatment, dead skin cells were reduced to the level of the normal group.
Moreover, epidermis thickness was increased in the DNCB-induced dermatitis control group, with thick epidermis skin damages that extended down to the dermis. The infiltration of inflammatory cells in the dermis was significantly improved in the 10 mg/mL treatment group and was decreased to the level of the normal group (Fig. 3C).
>
Serum IgE production change examination
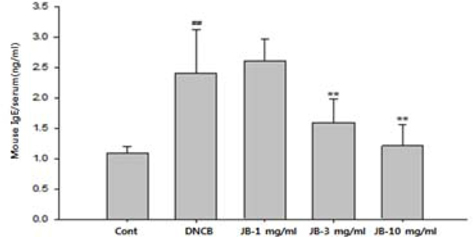
Elevated serum IgE level is known to be an immunological indicator of AD. Specifically, the level of IgE is known to be in proportion to the clinical severity of AD patients. IgE is reported to produce histamine by combining with white blood cells and skin mast cells and then developing allergic reactions via reaction with invading antigens (Baugh and Bucala, 2001). When silkworm feces extracts in various concentrations were applied to AD-induced mice, IgE content tended decrease in a dose-dependent manner compared to that of the DNCB treatment group and was significantly decreased in the silkworm feces extracts 3 and 10 mg/mL treatment groups (Fig. 4). Skin allergic reaction leads to hyperpermeability of expansion blood vessels, increased mucous, edema, and inflammation of mucous from various chemicals, due to chemical transport of histamine or T-lymphocytes that are released by the reaction of IgE with antigens (Meyer, 2003). Histamine is a major cause of uredo in AD and elevated histamine is reported to be found in atopy patients compared to normal individuals. Skin forms a boundary for the body against the external environment and has chances of contact with numerous antigens. Once AD occurs, people scratch their skin due to the decreased threshold regarding skin hypersensitivity reaction and uredo. The scratching stimulation and inflammatory reactions secrete cytokines in the dead skin cells which aggravates the inflammatory reaction so that it induces transformation of the dead skin cell layers and activates immune cells. Activation of the immune cells induces an increase in IgE production and antibody reaction and increases activity of the IgE-dependent free histamine thereby promoting histamine secretion. Histamine is reported to induce infiltration of eosinophils, acute hypersensitivity reaction, and uredo (Budde
>
Inflammatory cytokine mRNA expression changes in skin tissues
Tumor necrosis factor alpha (TNF-α) is known to be an important chemical mediator involved in inflammation processes. Interleukin-1beta (IL-1β) and interleukin-4(IL-4), inflammatory cytokines originated from macrophages, are also known to be representative mediators of tissue damage inflammatory reactions that induce MMP (matrix metalloproteinase) activation, the main enzyme involved in the destruction of the extracellular matrix and inflammation (Maeda and Yanagihara, 2001).
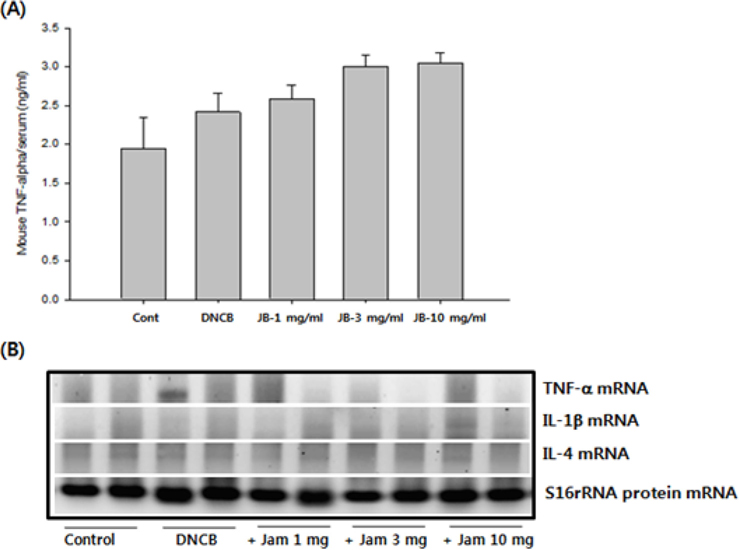
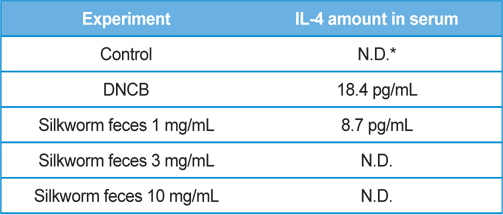
As a result of quantifying TNF-α, a representative inflammatory cytokine, using the serum samples obtained at the end of dermatitis experiment, there were no significant differences in the DNCB treatment group and silkworm feces extracts treatment group at various concentrations compared to the normal group (Fig. 5A). Levels of mRNA expression for TNF-α, interleukin-1beta (IL-1β), and interleukin-4 (IL-4) did not show significant differences (Fig. 5B). Given the results, silkworm feces extracts are believed to have no influence in the levels of inflammatory cytokines in the skin tissue itself, but quantification of blood IL-4 resulted in maximum 18 pg/ mL of IL-4 detected in the DNCB treatment group. Also, when silkworm feces extracts were applied, IL-4 was decreased but was not detected when 10mg/mL of the silkworm feces extracts were applied, indicating that silkworm feces extracts can inhibit expression of cytokines (Table 1). IL-4 is a cytokine that divides T cells into Th2 and produces IgG1 and IgE by stimulating B cells. This indicates that the increase of IL-4 and IgE are correlated, which was in agreement with the results of previous studies, which suggested that increased IgE levels increases infiltration of inflammatory cells and mast cells around the small blood vessels (even in skin tissue specimens) thereby developing dermatitis such as atopy and contact allergies (Leung, 2000; Leung
[Table 1.] Production amounts of IL-4 in serum.
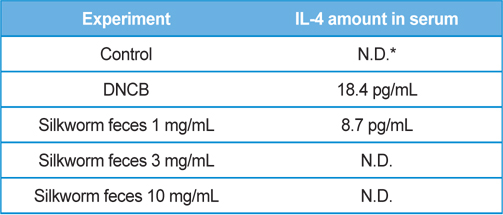
Production amounts of IL-4 in serum.
Therefore, the results of this study suggest that silkworm feces extracts can be developed as a raw material for medicines and functional cosmetics that improve the symptoms of atopic dermatitis. However, the component (or combination of components) of the tested silkworm feces extracts that is effective in relieving the symptoms of atopic dermatitis is yet to be determined. Therefore, further studies need to be performed to identify the effective components that improve atopic dermatitis symptoms and their mechanisms of action.