Size reduction (sieving) is the method by which solid pieces of food are reduced in size by grinding, compression, or impact. When force is applied to a food the resulting internal strains are first absorbed and cause deformation of the tissues. If the strain does not exceed a critical level, the tissues return to their original shape when the stress is removed, and the stored energy is released as heat. Size reduction is used to control the textural or rheological properties of foods, and improves the efficiency of mixing, extraction, and heat transfer. Dry foods have sufficiently low water to permit storage up to several months after grinding without substantial changes in nutritional value, taste, or microbial safety (Fellows, 2009).
Industrial waste and mining can be sources of heavy metal pollution in aquatic environments (Gumgum et al., 1994) that can be transferred to marine organisms. Fish and other marine invertebrates are considered as bio-indicators of metal concentrations in aquatic ecosystems (Domingo et al., 2007), because they can concentrate large amounts of metals in their tissues (Yilmaz et al., 2007). It is important to determine the concentrations of heavy metals in commercial fish in order to evaluate the possible health risks of fish consumption by humans (Yilmaz et al., 2007). The accumulation of heavy metals in human tissues can result in chronic illness and cause potential damage to the population.
Microbial safety is an important indicator of protein qualityand storage tolerance. There are many techniques to reduce the microbial content of foods. For the past two decades, high-pressure carbon dioxide (HPCD) has been used as a nonthermal pasteurization technique for food (Spilimbergo et al., 2002). In the HPCD technique, food is contacted with supercritical carbon dioxide (SC-CO2) for a certain amount of time in a batch, semi-batch or continuous manner. SC-CO2 has the ability to diffuse through solids like a gas, and to dissolve materials like a liquid. Additionally, it can readily change density with minor changes in temperature or pressure. The SC-CO2 technique presents advantages over high hydrostatic pressure (HHP) because of the milder conditions required. In addition to the environmentally benign nature of the SC-CO2 process (CO2 is nontoxic), the CO2 pressures applied for preservation are much lower (generally below 10 MPa) than the hydrostatic pressures employed in HHP (300-600 MPa), making it easier to control pressure in the SC-CO2 technique (Spilimbergo et al., 2002). Alcohol also has as antimicrobial properties. The bactericidal activity of alcohol is due to several factors: disruption of membrane structure or function (Fried and Novick, 1973; Halegoua and Inouye, 1979; Barker and Park, 2001; Silveira et al., 2004); interference with cell division, affects on steady-state growth (Fried and Novick, 1973); variations in fatty acid composition and protein synthesis (Chiou et al., 2004); inhibition of nutrient transport via membrane-bound ATPases (Bowles and Ellefson, 1985); alteration of membrane pH (Bowles and Ellefson, 1985; Terracciano and Kashket, 1986) and membrane potential (Terracciano and Kashket, 1986); and a decrease in intracellular pH (Bowles and Ellefson, 1985; Huang et al., 1986; Terracciano and Kashket, 1986).
Refrigeration preserves food by slowing down the growth and reproduction of microorganisms and the action of enzymes that cause food to rot. Freezing is one of the most commonly used processes for preserving a wide range of foods, including prepared foods that would not have required freezing in their unprepared state.
The goal of this study was to determine the effectiveness of size reduction using various mesh sizes and to determine the solubility of mackerel muscle that has been freeze-dried, deoiled by SC-CO2, or roasted. In addition, we measure the concentrations of heavy metals and the inhibition of microbial growth under storage at different temperatures of SC-CO2-, hexane-, and ethanol-treated mackerel muscle to determine safety profiles.
Washed mackerel muscle samples were collected from F & F Co., Busan, Korea and transported to the laboratory on ice. Carbon dioxide (CO2, 99% pure) was supplied by KOSEM, Korea. All other reagents used in this study were of analytical or high performance liquid chromatography (HPLC) grade.
Raw mackerel muscle samples were freeze dried (EYELA FDV-2100 freeze dryer, Rikakikai Co. Ltd., Tokyo, Japan) for approximately 72 h. Dried samples was then crushed in a mechanical blender and stored at -20℃ for until analysis.
>
Supercritical carbon dioxide (SC-CO2) treatment
We used a small-scale of supercritical carbon dioxide extraction unit d to remove oil from freeze-dried mackerel muscle. This apparatus could be operated at pressures up to 700 bar. We placed 600 g freeze-dried mackerel muscle into the stainless steel extraction vessel tightly sealed it with a stainless steel cap. CO2 was then pumped at constant pressure through the extractor unit into the extraction vessel up to the desired pressure. A back-pressure regulator was used to control the CO2 pressure. The extraction temperature was maintained by a water bath connected to the extraction vessel. The experiment was carried out at 55℃ and 400 bar for 2 h to completely remove oil from the muscle. Finally, the remaining oil-free muscle residues (deoiled residues) were again crushed in a mechanical blender and stored at -20℃ for further analysis.
We used hexane and ethanol to remove oil from freezedried crushed mackerel muscle. We placed250 g mussle tissue in a beaker with 1,250 mL of either hexane or ethanol and stirred at 300 rpm using a magnetic stirrer for 24 h at 45℃. After extraction, the solvent was separated by filtration, and the remaining residues were dried at 40℃ for 2 h. The lipidfree residues were then collected, crushed again in a mechanical blender and stored at -20℃ until further use and analysis.
We roasted 200 g freeze-dried crushed mackerel muscle in an 80℃ oven for 6 h. The sample was then cooled at room temperature, crushed in a mechanical blender, and stored at -20℃ for further analysis.
Freeze-dried mackerel muscle, roasted muscle, and solvent- or SC-CO2-deoiled muscle were sieved through mesh sizes of 300 µm, 500 µm, 710 µm and 1,000 µm, respectively. Sieve and percent mass retention were calculated according to the modified method of Fellows (2009).
>
Determination of percent solubility
We mixed 2 g of each sieved sample from freeze-dried, roasted or deoiled mackerel muscle with water, hexane, or ethanol at a 1:10 ratio and stirred at 400 rpm for 1 h at room temperature. The solutions were then separated by filtration, and the remaining residues were dried and weighed. The percent solubility was determined as:
Solubility (%) = (weight of initial sample - weight of residues after reaction) × 100
>
Measurement of heavy metal contents
Metals contents were measured in mackerel muscle (sieved at 710 µm) of each treatment according to the method of Ackacha et al. (2010) with some modifications. We dissolved 1 g of each sample in 10 mL nitric acid, and heated it at 130℃ for 5 min until it was fully dissolved, and filtered the sample. We then transferred the sample to a 100 mL volumetric flask filled with de-ionized water. We used inductively coupled plasma mass spectrometry (ICP-MS; Elan 6100, Perkin-Elmer, USA) to measure the concentrations of arsenic (As), cadmium (Cd), lead (Pb), iron (Fe) and zinc (Zn), and we used an inductively coupled plasma optical emission spectrometer (ICP-OES; Optima 7300DV, Perkin-Elmer, USA) to measure mercury (Hg).
We counted the number of microbial colonies in the different treatment samples of mackerel muscle (710 µm sieve size) using the plate count method (Gilbert et al., 2000; Cappuccino and Sherman, 2002). We mixed 1 g of sample with 5 mL 0.85% NaCl solution (saline) and vortexed for 2 min to dissolve. We then filtered the solution through filter paper and diluted in saline at dilutions of 10-1, 10-2, 10-3, or 10-4. We used nutrient agar (Merck KGaA, Darmstadr, Germany) that was sterilized at 121℃ for 15 min as growth medium for bacterial strains. We then poured the agar into sterile glass petri dishes and allowed to set. We inoculated the plates with 1 mL of diluted samples and incubated at 37℃ for 24 h, after which we counted the number of bacterial colony-forming units (CFU) and estimated the total bacterial concentration as log(CFU/mL).
All experiments were carried out in triplicate. Data are expressed as the means ± standard deviation (SD). Significant differences (
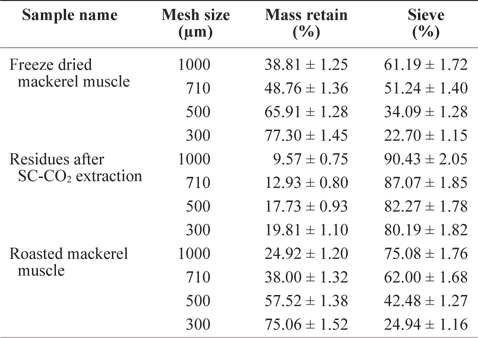
The residue size of mackerel muscle became smaller after removing oil using SC-CO2 (Table 1). The sieve percentages for de-oiled muscle residues sieved at 1,000 µm and 300 µm mesh were determined to be 90.43% and 80.19%, respectively, which were higher than those values for freeze-dried mackerel muscle (61.19% and 22.70%) and roasted mackerel (75.08% and 24.94%) using the same mesh sizes (Table 1). This is because after removing the oil, the residues were less sticky, allowing greater ability to pass through the sieve. The low sieve percentages for roasted mackerel muscle compared with freeze-dried mackerel muscle may be because the roasted residues were thoroughly dried by the heat treatment.
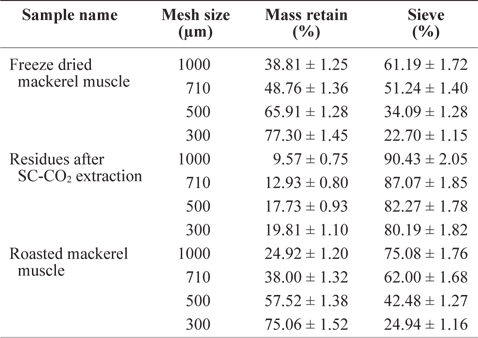
Sieve percentage determination of differently treated mackerel muscle residues using various mesh size
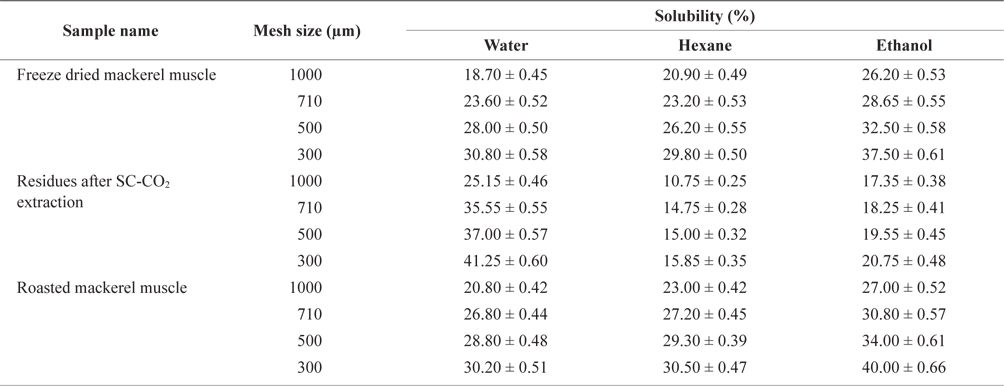
The solubility percentage of freeze-dried, SC-CO2 extracted, and roasted mackerel muscle samples varied for different size residues and after sieving with different solvents (Table 2). The highest solubility value for deoiled mackerel (41.25%) was found when the muscle was sieved in water through a 300 µm sieve. Due to oil separation, the solubility of tissue residues was higher in water. In contrast, the highest solubility value for roasted mackerel muscle were found when using hexane (30.50%) and ethanol (40.00%) with a 300 µm sieve size. Therefore, the solubility of deoiled residues was higher in water, and non-deoiled residues were more soluble in organic solvents.
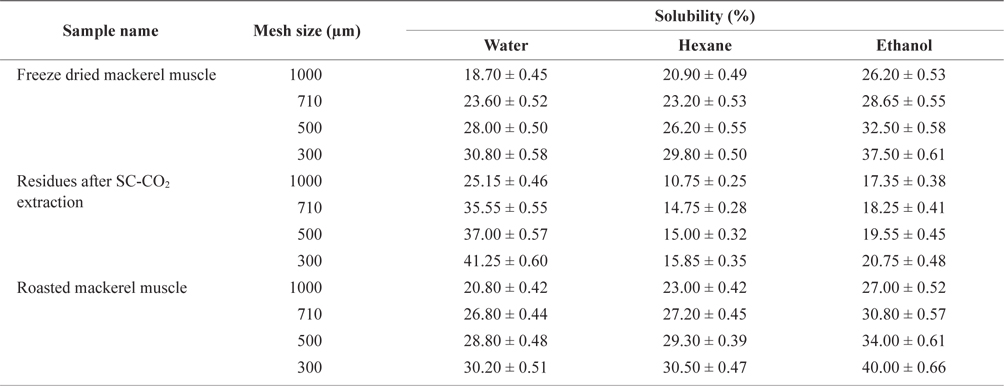
Solubility percentage determination of differently treated mackerel muscle residues using various solvent
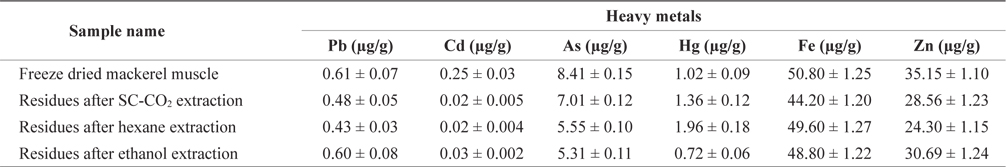
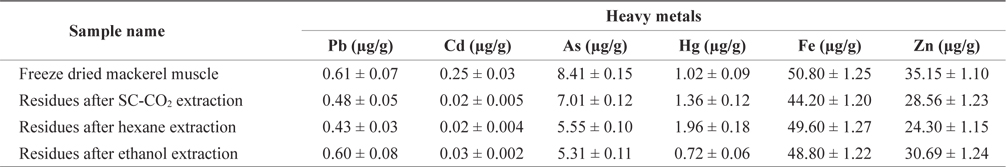
Maximum permitted level of some heavy metals in fish enforced by Australia and Singapore showed in Table 3. Lead(Pb) is known to reduce cognitive development and intellectual performance in children and to increase blood pressure and cardiovascular disease in adults (Commission of the European Communities, 2002). The Pb concentration in freezedried mackerel muscle was 0.61 µg/g, and this concentration decreased after treatment (Table 4). The Pb concentrations of mackerel muscle treated with SC-CO2, hexane, and ethanol were 0.48, 0.43 and 0.60 µg/g, respectively. According to the Australia New Zealand Food Authority (2002) and FAO guidelines (Sivaperumal et al., 2007), the allowable level of Pb in fish is 0.5 µg/g. The Pb levels in our SC-CO2- and hexane-treated muscles were less than these recommended levels. The Pb concentrations in freeze-dried and ethanol-treated muscle were also lower than the 2 µg/g level recommended by the government of Singapore (1990).
[Table 3.] Maximum permitted level of some heavy metals in fish enforced by Australia and Singapore

Maximum permitted level of some heavy metals in fish enforced by Australia and Singapore
[Table 4.] Heavy metals content of differently treated mackerel muscle residues
Heavy metals content of differently treated mackerel muscle residues
Cadmium (Cd) is toxic to humans and it can accumulate in the body, where it may induce kidney dysfunction, skeletal damage and reproductive deficiencies. The greatest concentration of Cd was found in freeze-dried mackerel muscle (0.25 µg/g) and the lowest concentrations in SC-CO2- and hexane-treated muscle (0.02 µg/g). According to Nauen (1983) guidelines, the maximum permissible Cd levels are 0.05-5.5 µg/g. The Cd levels in all our muscle samples were within this range.
Arsenic (As) toxicity is not fully understood, but its possible effects include hair loss, dermatitis, diarrhea and other gastrointestinal symptoms, fatigue, headaches, confusion, muscle pains, red and white blood cell problems, neurologic symptoms, and liver and kidney damage. The As concentrations in mackerel muscle that was freeze-dried or SC-CO2-, hexane, or ethanol-treated were 8.41, 7.01, 5.50 and 5.31 µg/g, respectively. The As concentration on treated and non-treated mackerel muscle was much higher than the permissible limits set by the Australia New Zealand Food Authority (2.0 µg/g; 2002) and the government of Singapore (1.0 µg/g; 1990).
According to the guidelines of the FAO/WHO (1972), the Australia New Zealand Food Authority (2002) and the Singapore government (1990), the permissible mercury (Hg) concentration in fish is 0.5 µg/g but our freeze-dried and SC-CO2-, hexane- and ethanol-treated mackerel muscle samples had Hg concentrations of 1.02, 1.36, 1.96 and 0.72 µg/g, respectively, which were higher than the acceptable levels. The FAO/WHO (1972) also reported acceptable Hg values for swordfish, tuna, and halibut as 0.20-1.5 l µg/g.
Zinc (Zn) is involved in most metabolic pathways in humans. It is widespread in living organisms due to its biological significance. Zn deficiency can lead to loss of appetite, growth retardation, skin changes and immunological abnormalities (Malakootian et al., 2011). According to the Food Codex (Maff, 1995), the permissible Zn level for fish is 50 µg/g. The highest Zn concentration in freeze-dried mackerel muscle was 35.15 µg/g and this concentration decreased slightly after treatment. The United States Environmental Protection Agency (US-EPA) and the European Commission (EC) have not set any standards or limits for zinc concentrations (Alimentarius, 1994; Ashraf, 2006).
Iron (Fe) concentrations in freeze-dried, SC-CO2-, hex- ane- and ethanol-treated mackerel muscle were 50.80, 44.20, 49.60 and 48.80 µg/g, respectively. We found no reports on the maximum safe levels of Fe in fish tissues. Turkmen et al. (2006) reported that Fe concentrations in fish muscle ranged from 8.87-18.80 µg/g, respectively. Essential metals, such as Fe and Zn, showed higher concentrations in mackerel muscle, presumably due to their function as co-factors for enzyme activation that causes tight regulation of their homeostatic levels in fish (Yilmaz, 2009).
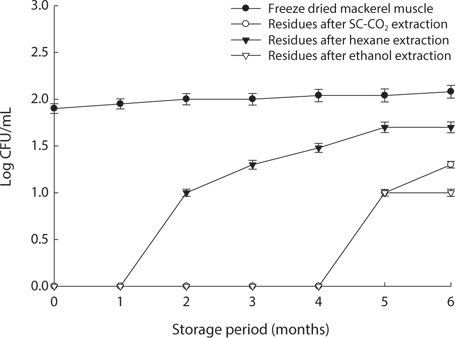
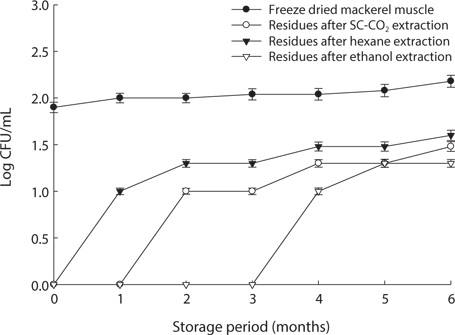
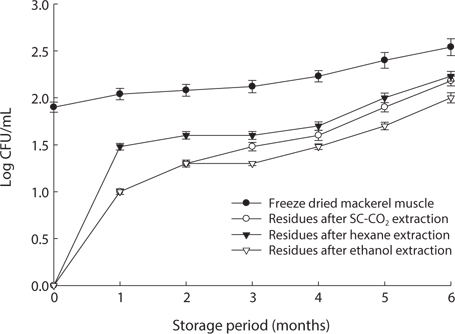
We found changes in bacterial plate counts in freeze-dried, SC-CO2-, hexane and ethanol-treated muscle after storage for six months at -20℃, 5℃ and 25℃ (Figs. 1-3). During storage at -20℃, freeze-dried mackerel muscle showed bacterial colonies of 1.90 log CFU/mL, and there were no drastic changes after 6 months. However, SC-CO2- and ethanol-treated mackerel muscle showed no bacterial colonies for up to 4 months and very few colonies after 5 or 6 months of storage, bacterial colonies in freeze-dried mackerel muscle were 2.18 and 2.54 log(CFU/mL) at 5℃ and 25℃, respectively, which were higher than the number of colonies at -20℃ (2.08 log(CFU/ mL)). Microbial growth was very slow after treating the mackerel muscle under different storage conditions, compared with freeze-dried mackerel muscle. Among the treated mackerel muscle samples, bacterial colonies were very low in those treated with SC-CO2 and ethanol. Lin et al. (1992a, 1993) reported that SC-CO2 penetrates cells and accumulates to a critical density, after which it removes intracellular constituents, such as phospholipids and hydrophobic compounds, which disturb or alter the structure of the biomembrane and balance in the biological system, thus promoting microbial inactivation. Many authors have reported that ethanol has microbial inactivating properties (Dabbah et al., 1970; Smith et al., 1987; Smith et al., 1994; Corcuff et al., 1996; Daifas et al., 2000). Therefore, SC-CO2- and ethanol-treated mackerel muscle are good methods for long term storage.