The
The relative proportions of EPA and DHA are equally important to the total
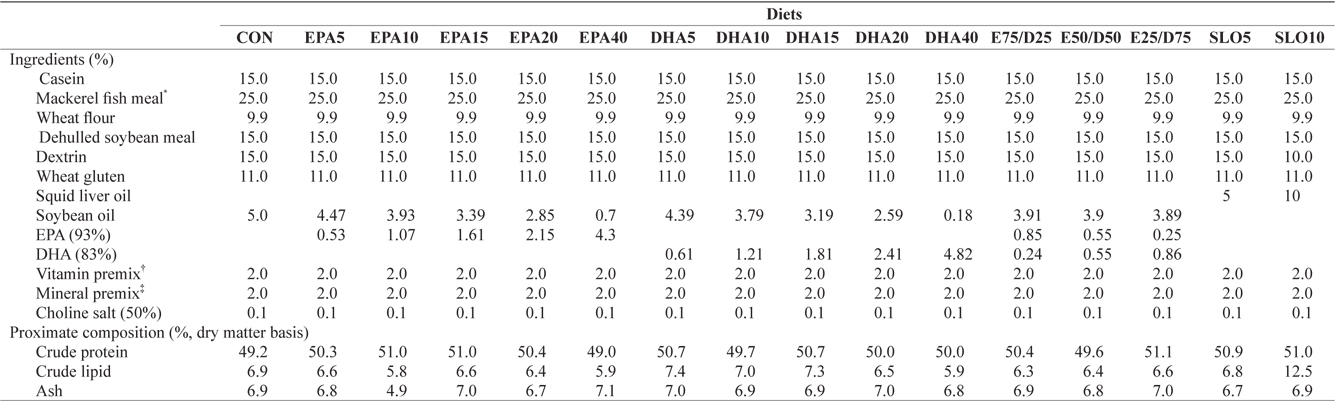
Ingredients and proximate compositions of the experimental diets are presented in Table 1. Fish meal and casein were used as the main protein sources and wheat flour was applied as the primary carbohydrate source. Sixteen experimental diets were prepared, which contained five levels of pure EPA (0.5%, 1.0%, 1.5%, 2.0%, and 4.0% of dry matter) and DHA (0.5%, 1.0%, 1.5%, 2.0%, and 4.0% of dry matter), three ratios of EPA/DHA (75/25, 50/50, and 25/75), two levels of squid liver oil (5% and 10%) and a control diet containing 5% soybean oil. Crude protein and lipid levels of the experimental diets were maintained at 50% and 6.5%, respectively, based on the results of a previous study (Lee at al., 2000). The contents of
[Table 1.] Ingredients and nutrient of the experimental diets
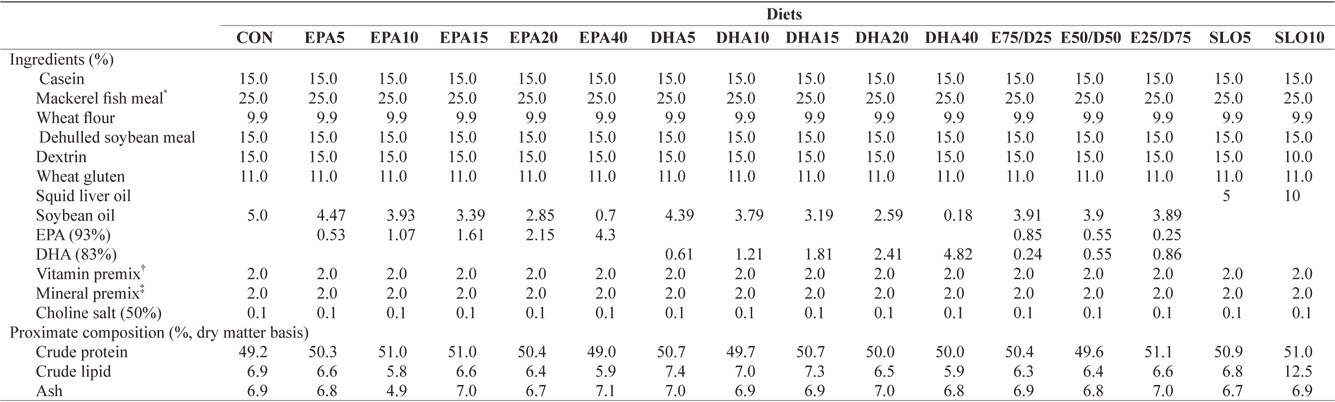
Ingredients and nutrient of the experimental diets
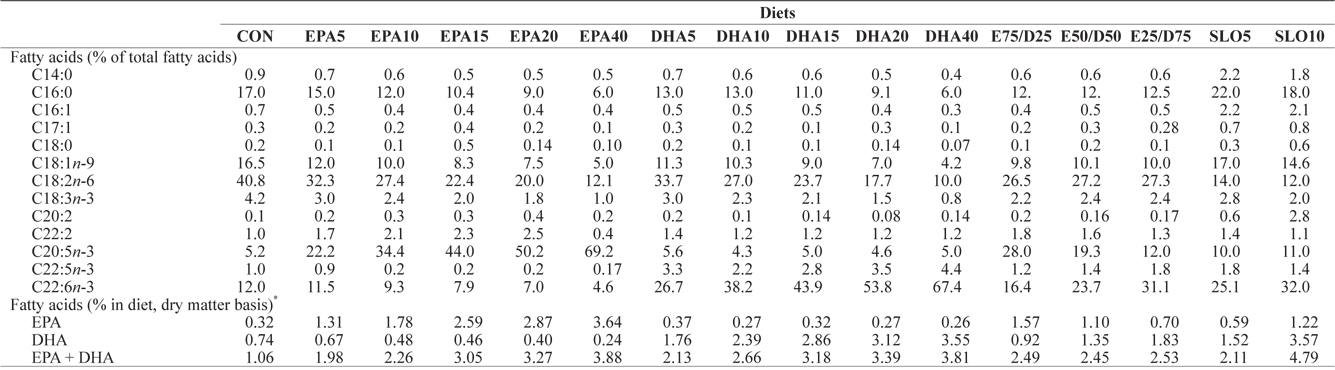
[Table 2.] Major fatty acids composition (% of total fatty acids) of the experimental diets
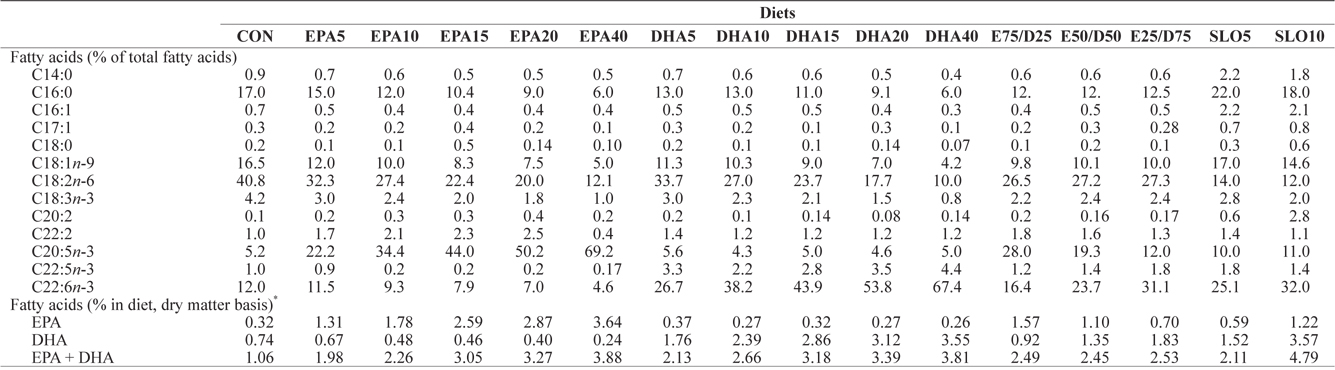
Major fatty acids composition (% of total fatty acids) of the experimental diets
>
Experimental fish and feeding trial
Juvenile olive flounder were obtained from a local farm in Taean, Korea. Fish were transported to the experimental facilities, acclimated to the experimental conditions, and fed with a commercial diet for 2 weeks prior to the start of the feeding trial. Juveniles (average weight, 9.7 ± 0.3 g) were randomly distributed into 48 tanks (50 L water volume) at a density of 20 fish per tank. Three replicate groups of fish were hand-fed to apparent satiation twice a day (09:00 and 17:00 for 6 days per week) for 8 weeks. Water temperature was 16.7 ± 1.8℃ and the photoperiod followed natural conditions during the feeding trial. Records were kept of the daily feed consumption, mortalities and feeding behavior of each tank.
>
Sample collection and chemical analysis
At the end of the feeding trial, all of the fish in each tank were collectively weighed after anesthetizing with tricaine methane sulfonate (MS222; Sigma-Aldrich, St. Louis, MO, USA) at a concentration of 100 ppm after starvation for 24 hours. Total length, body weight, liver weight and intestine weight of five fish from each tank were measured. Five fish from each tank were taken and pooled to determine whole body composition. The dorsal muscle and liver of 10 fish from each tank were removed and stored at –75℃ for subsequent proximate analysis. The crude protein content was determined using the Kjeldahl method with the AutoKjeldahl System (Buchi, Flawil, Switzerland). The crude lipid content was determined by the ether-extraction method using a Soxhlet extractor (VELP Scientifica, Milano, Italy). The moisture content was determined with a dry oven (105℃ for 6 h), and the ash content was determined using a muffler furnace (600℃ for 4 h). Lipid for fatty acid analyses was extracted with a mixture of chloroform and methanol (2:l, v/v) according to the methods described by Folch et al. (1957), and fatty acid methyl esters were prepared by trans-esterification with 14% BF3-MeOH (Sigma-Aldrich). Fatty acid methyl esters were analyzed using a gas chromatograph (Clarus 600; PerkinElmer, Shelton, CT, USA) with a flame ionization detector, equipped with an SP-2560 capillary column (L × I.D. 100 m × 0.25 mm; film thickness 0.20 μm; Supelco, Bellefonte, PA, USA). Injector and detector temperatures were both 240℃. The column temperature was programmed from 140℃ to 240℃ at a rate of 5℃/min. Helium was used as the carrier gas. Fatty acids were identified by comparison with retention times of the standard fatty acid methyl esters (PUFA 37 component FAME Mix; Supelco).
The data were subjected to one-way analysis of variance (ANOVA) using SPSS version 19.0 (SPSS Inc., Chicago, IL, USA). Significant differences (
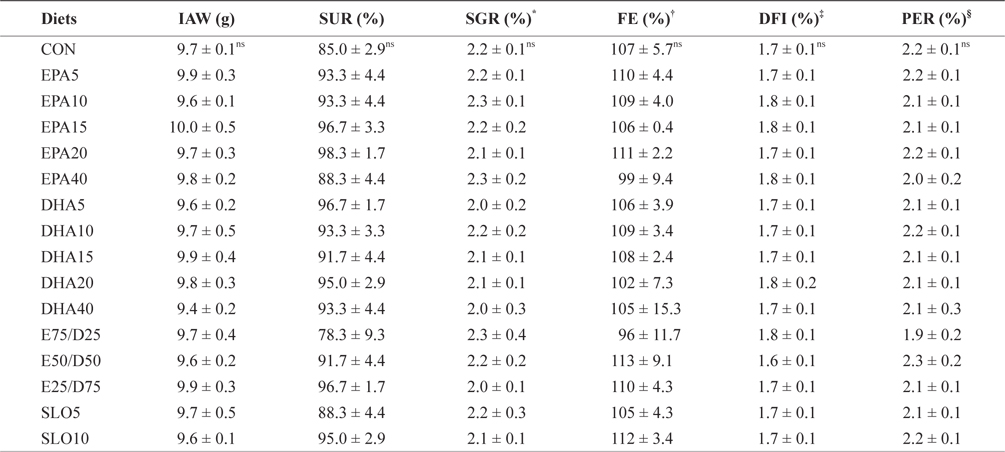
The growth performances of fish fed with the experimental diets for 8 weeks are shown in Table 3. Increasing the EPA and DHA levels in diets up to 4.0% had no significant effect on survival and growth performance of the juvenile olive flounder. Also, no significant differences were found in the specific growth rate, feed efficiency, protein efficiency ratio, or daily feed intake as dietary EPA/DHA was increased from 25% to 75% (
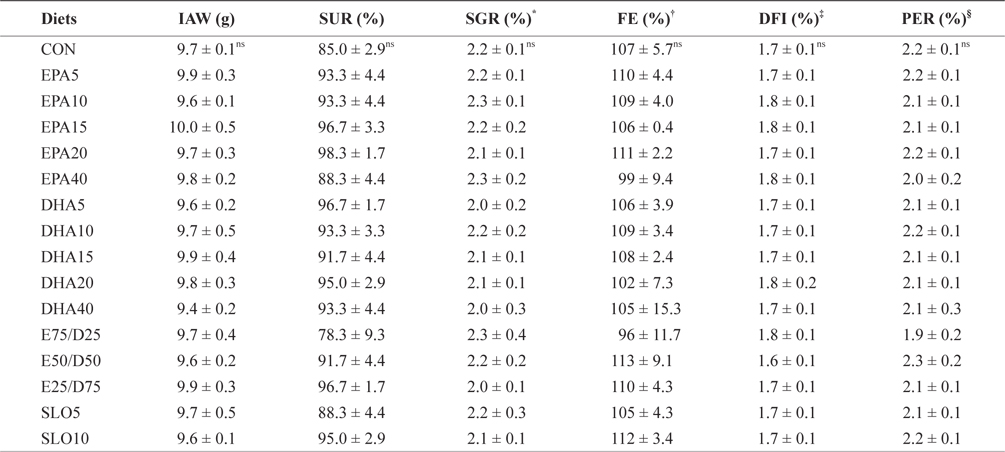
Growth performance and feed utilization of juvenile olive flounder fed the experimental diets for 8 weeks
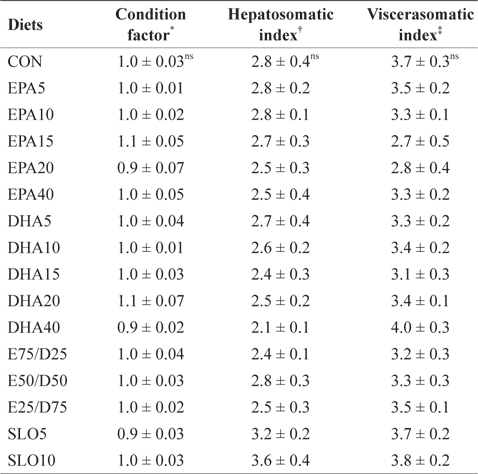
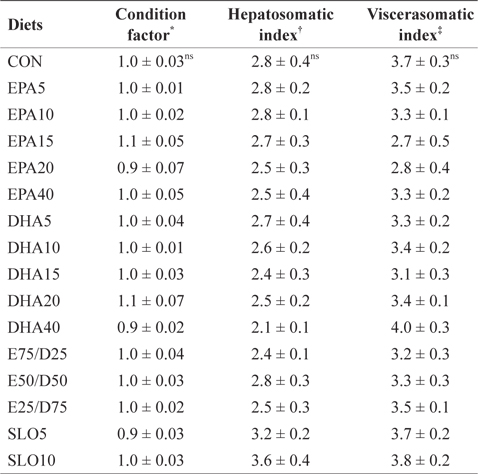
Morphological parameters of juvenile olive flounder fed the experimental diets for 8 weeks
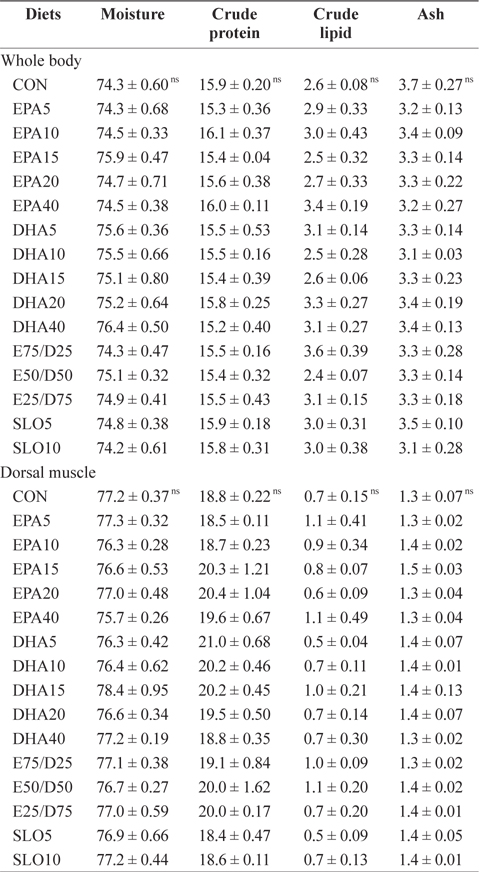
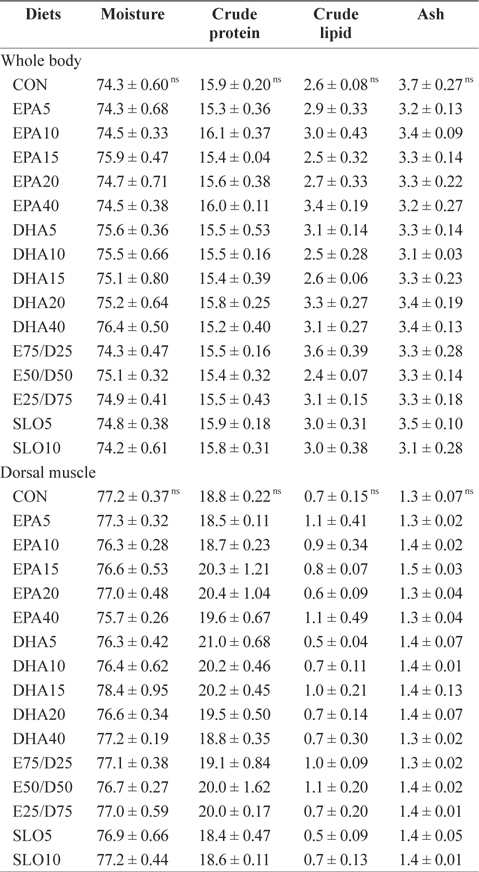
Chemical composition (%, wet weight basis) of the whole body and the dorsal muscle of juvenile olive founder fed the experimental diets for 8 weeks
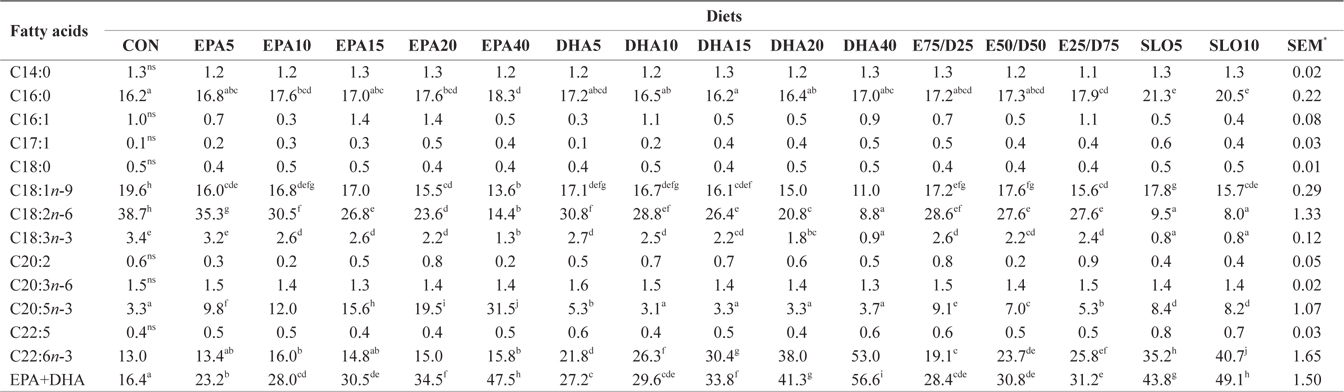
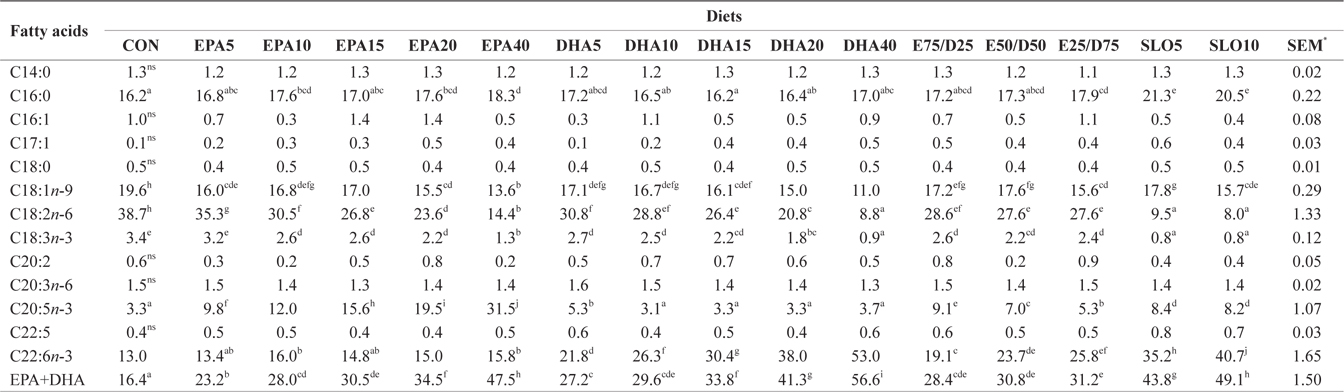
Major fatty acids composition (% of total fatty acids) of dorsal muscle in juvenile olive flounder fed experimental diet for 8 weeks
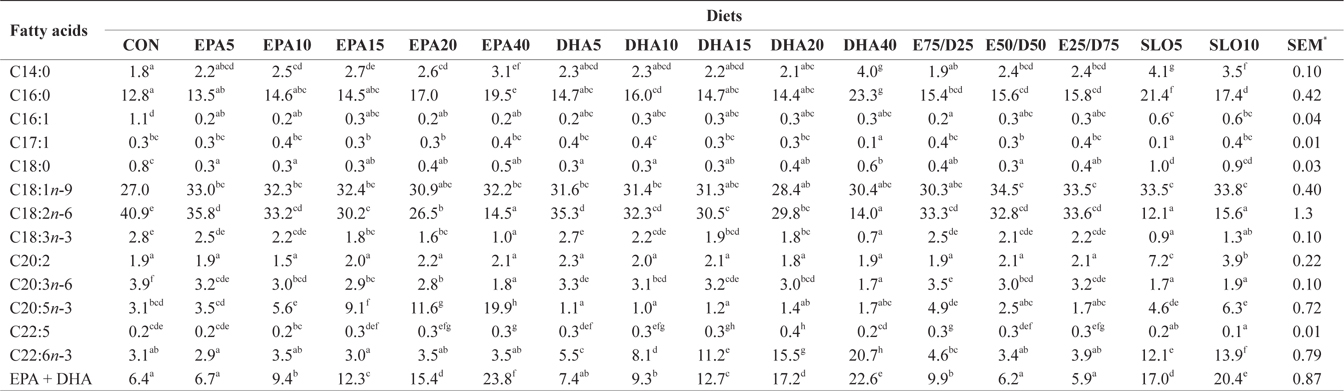
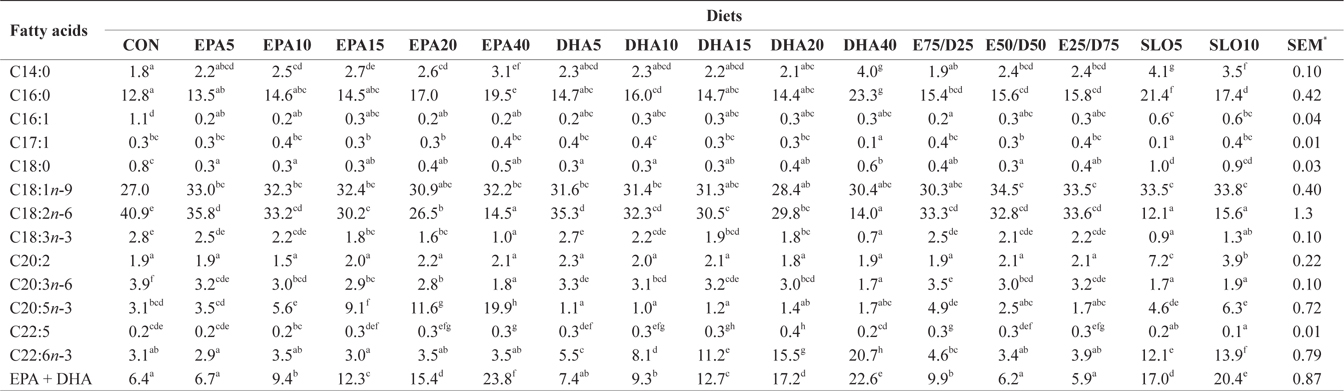
Major fatty acids composition (% of total fatty acids) of liver in juvenile olive flounder fed experimental diet for 8 weeks
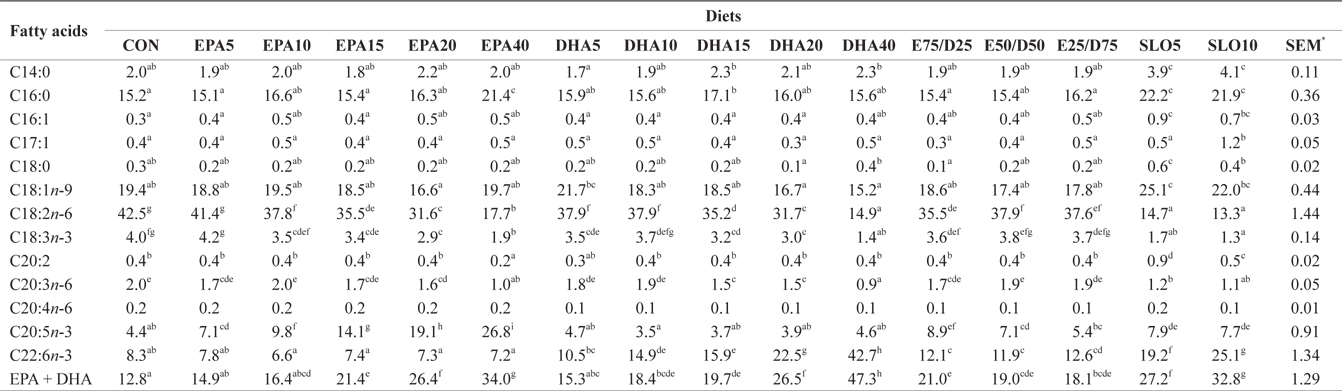
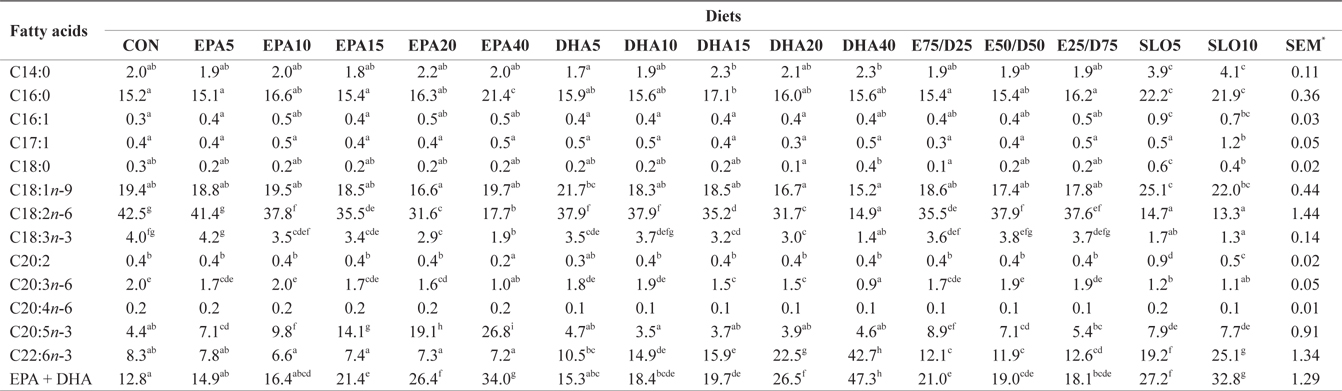
Major fatty acids composition (% of total fatty acids) of whole body in juvenile olive flounder fed experimental diet for 8 weeks
The results of the present study indicate that different levels of neither EPA nor DHA had significant effects on the survival, growth performance, morphological parameters, or body composition of juvenile olive flounder. These results indicate that juvenile olive flounder can tolerate a wide range (1.06-4.79%) of EPA and DHA variation in their diet. Similar results have been obtained from previous studies; olive flounder larvae were able to grow and survive past metamorphosis on
The different responses of fishes to excessive
The essential fatty acid values of EPA and DHA were compared in juvenile fish by feeding the fish diets that contained various ratios of EPA to DHA. No correlation between dietary EPA/DHA ratios and fish growth performance was observed in this study. These results were similar to those from previous studies on turbot and flounder (Dickey-Collas and Geffen, 1992; Furuita et al., 1998, 1999). However, other studies on larval yellowtail (Furuita et al., 1996), larval and juvenile striped jack (Takeuchi et al., 1996) and larval flounder fed a microdiet (Watanabe and Kiron, 1994) have shown that DHA is superior to EPA as an essential fatty acid. These observations indicated that the requirement for DHA in the larval stage is higher than that of juveniles. The results of the present experiment indicate that adding either EPA, DHA, or a combination thereof has no significant effect on fish growth. However, in an earlier study by Kim and Lee (2004), a combination of EPA and DHA in the diet was more effective on the growth of juvenile flounder than the use of EPA only. The difference in results may have arisen when using diets that contained soybean oil masked the effects of EPA and DHA (Kim et al., 2012).
In this experiment, the liver, muscle and whole body fatty acid compositions were reflective of the respective dietary fatty acids. However, the DHA content in muscle was greater than its respective level in the diet, regardless of the dietary treatment. Similar results were also described for flounder in multiple studies (Kim and Lee, 2004; Kim et al., 2012). The selective deposition of DHA may be related to the high specificity of a synthesis enzyme, such as 1-lysophosphatidylacyl CoA transferase for DHA. Also, the increased DHA/EPA ratio in muscle indicated a selective catabolism of EPA relative to DHA in fatty acid oxidative processes. The relative resistance of DHA to β-oxidation stems from the complex catabolic pathway of this fatty acid (Caballero, 2002; Mourente and Bell, 2006).
In conclusion, the results of this study suggest that juvenile olive flounder require a low level of dietary EPA for suitable survival and growth,