Hybridization between fishes is a practical and effective tool for improving the production of fish; the technique has also been used with domestic animals since prehistoric times. Hybrid striped bass
Red sea bream
Dietary protein and lipid levels are essential factors for efficient fish production (National Research Council, 1993). It is known that some hybrids have similar or different dietary protein and lipid requirements compared with their parents (Brecka et al., 1995; Twibell et al., 2003). Moreover, there are some suggestions that the hybrids showed heterosis in growth, survival and disease tolerance, distinguishable from their parent species (Logan, 1968; Blanc and Chevassus, 1982; Tuncer et al., 1990; Brecka et al., 1995; Moreau and Pauly, 1999; Harel and Place, 2003).
Given this background, this investigation was conducted to determine a suitable dietary protein/lipid ratio for early F1 juveniles, which would be useful information for further promoting and developing the F1 aquaculture industry in East China and Korea.
The F1 were produced artificially by cross-breeding of mature female RSB of the Kinki University strain (4 years old, 4.5 ± 0.4 kg,
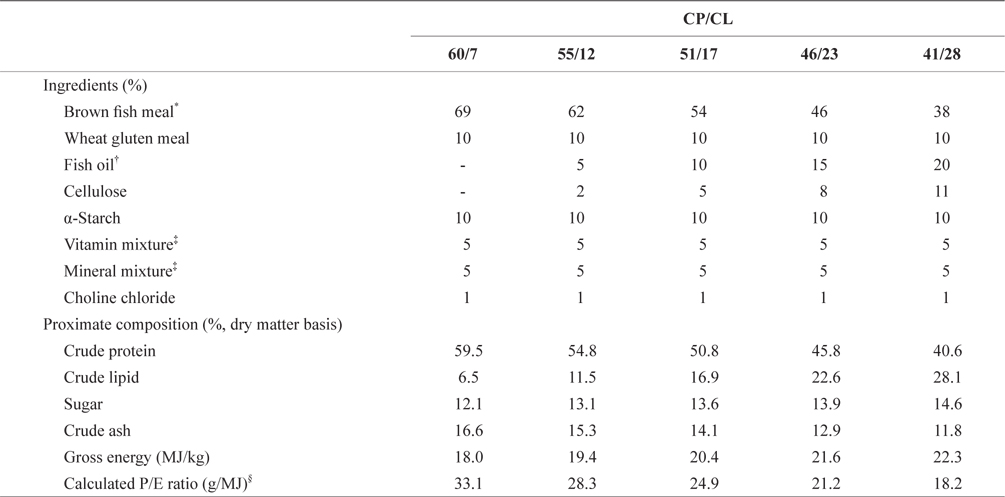
Five diets were prepared with various ratios of crude protein/lipid (CP/CL)60/7, 55/12, 51/17, 46/23, and 41/28as shown in Table 1. Brown fish meal (crud protein and lipid contents were ca. 69.2 and 9.4%, respectively) and wheat gluten and fish oil were supplied as the main protein and lipid sources, respectively. All experimental diets were prepared by mixing the ingredients appropriately and adding 30% tap water externally, and were then pelletized using a laboratory pellet machine. The pellets were freeze-dried and sieved to obtain two particle sizes for the two separate trials using F1 and RSB of different sizes. The pellet particle size was 355-425 µm for smaller F1 and RSB juveniles, weighing 0.32 ± 0.01 g and 0.26 ± 0.01 g at 43 and 41 dAH, respectively. For larger F1 and RSB juveniles, weighing 3.67±0.06 and 4.03±0.07 g at 75 and 73 dAH, respectively, pellets of 1.9 mm in diameter were prepared and freeze-dried. The diets for both trials were prepared just before the start of each trial and stored in a freezer at ‒20℃ until used.
[Table 1.] Feed formula and proximate composition of the experimental diets
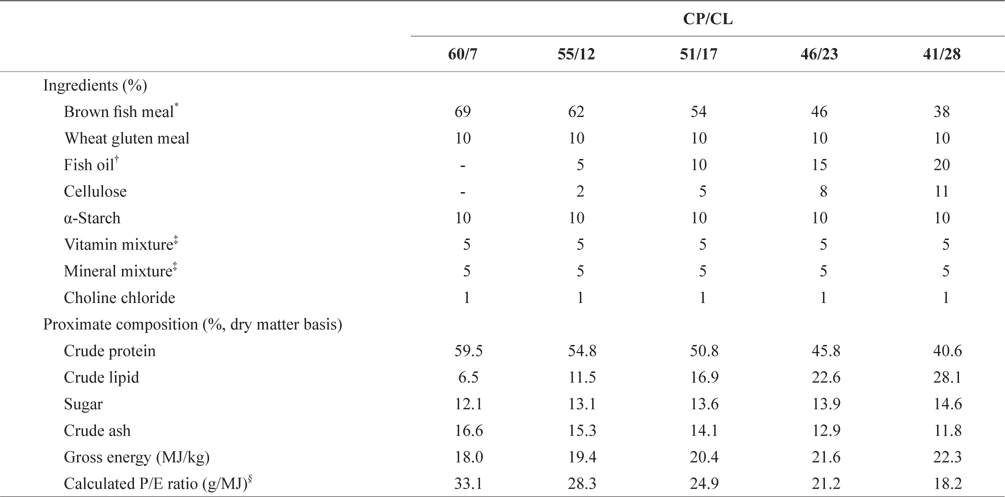
Feed formula and proximate composition of the experimental diets
There were two trials in this study. Both trials were conducted under a photoperiod cycle of 14-h light and 10-h dark.
In Trial 1, 50 smaller juveniles of F1 and RSB each were introduced into 40-L rectangular tanks. This trial was conducted in triplicate for each diet, and juveniles were fed four times daily until apparent satiation, at 08:00, 11:00, 14:00, and 17:00-h, for 2 weeks. Water temperature and DO were 20.6 ± 0.7℃ and 6.5 ± 2.1 mg/L, respectively.
In Trial 2, 20 larger juveniles of F1 and RSB each were introduced into 40-L rectangular tanks. This trial was also conducted in triplicate for each diet, and juveniles were fed three times daily, at 09:00, 13:00, and 17:00-h, until apparent satiation for 4 weeks. Water temperature and DO were 24.3 ± 2.2℃ and 7.6 ± 1.7 mg/L, respectively.
At the beginning and end of each trial, juveniles were weighed and sampled after fasting for 24-h. Growth performances of the fishes were evaluated at the end of each trial and compared among the treatments. Growth performance was estimated using daily feeding rate, survival, specific growth rate (SGR), feed efficiency (FE), condition factor (CF), protein efficiency ratio (PER), and apparent protein, lipid, and energy retention efficiencies (PRE, LRE, and ERE). To evaluate the whole body proximate composition, 10 juveniles from Trial 1 and 5 from Trial 2 were collected from each tank. Also, six juveniles from each tank were sacrificed and dissected for various organ samples to measure hepatosomatic indices (HSI) and viscerosomatic indices (VSI). All samples were immediately transferred to a freezer (‒40℃) until analysis.
At the end of Trial 2, blood samples were obtained from the caudal aorta of three fishes per tank with heparinized syringes. Hematocrit levels and hemoglobin content (Hb) were determined using a microhematocrit method (Brown, 1980) and a commercial kit (Wako Pure Chemical Industries, Osaka, Japan), respectively. For assay of hepatopancreatic glutamateoxalacetate transaminase (GOT) and glutamate-pyruvate transaminase (GPT) activities, the hepatopancreas removed from juveniles was homogenized with nine volumes of deionized water (v/w) using a Potter-Elvehjem glass homogenizer. After centrifugation of the homogenate (10,000
The proximate compositions of the diets and whole bodies were assayed using Association of Official Analytical Chemists methods (1995). Sugar contents of diets were measured by the phenol-sulfuric acid method (Hodge and Hofreiter, 1962). The energy concentrations of diets and whole bodies were determined directly using an automatic oxygen bomb calorimeter (IKA-Werke, Staufen, Germany).
Data obtained from both trials were subjected to a two-way analysis of variance (ANOVA) to evaluate the effect of experimental diets, fish species, and interactions. Where significant differences were found, the means within and among the treatments were further compared using Duncan’s new multiple range test followed by one-way ANOVA at the
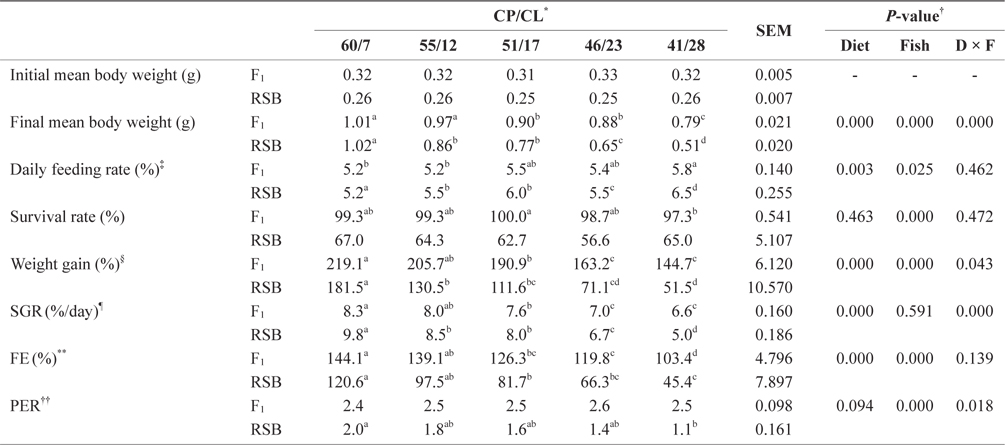
The growth performance data from Trial 1 are presented in Table 2. Two-way ANOVA showed that different dietary CP/ CL ratios had significant effects on final mean body weight, daily feeding rate, weight gain, SGR, and FE (two-way ANOVA,
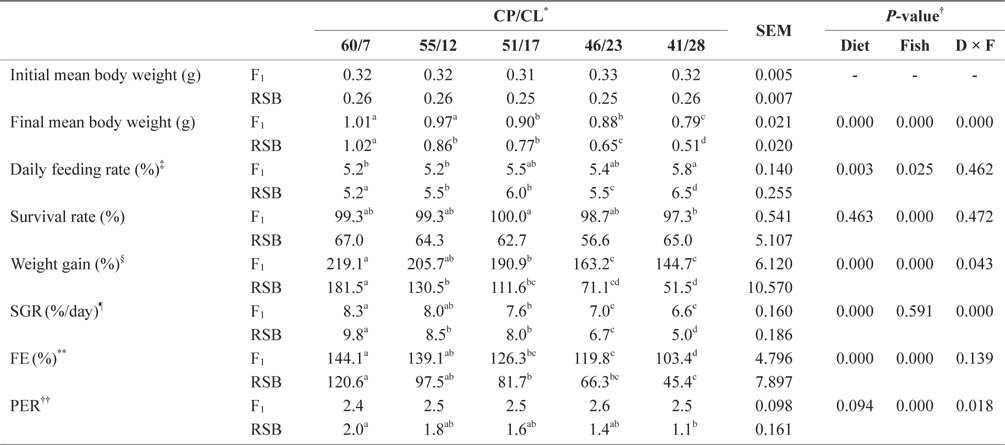
Effect of feeding diets with different protein/lipid levels on the growth performance of juvenile F1 and RSB (Trial 1)
In contrast, final mean body weight, weight gain, SGR, and FE showed decreasing trends with decreasing dietary CP/CL ratios for both species (
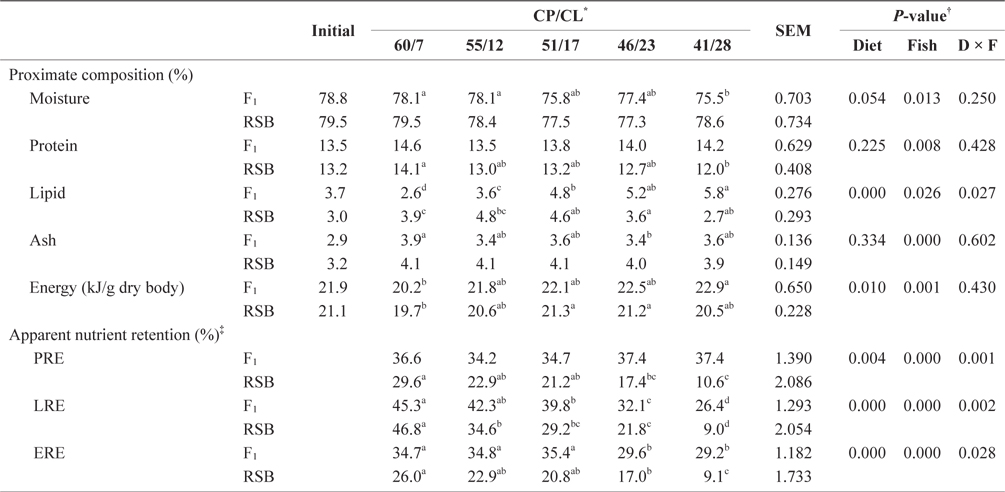
There was no difference in carcass proximate compositions between F1 and RSB at the start of the rearing trial (Table 3). At the end of Trial 1, two-way ANOVA showed significant differences in all proximate composition parameters, while both diet and species and their interaction had significant effects on apparent nutrients and energy retention (
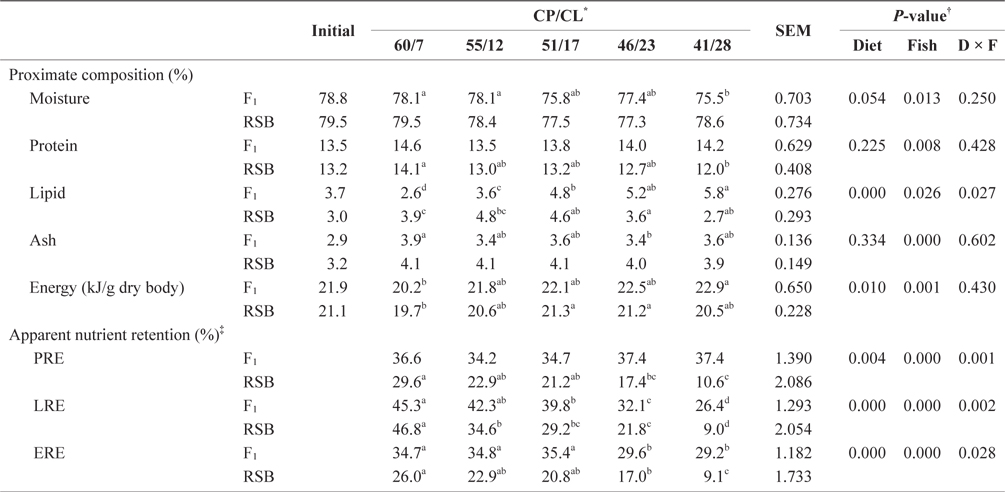
Carcass compositions and apparent protein, lipid and energy retentions of juvenile F1 and RSB fed experimental diets with different protein/ lipid level (Trial 1)
Although there was no difference in whole body protein content or PRE among the dietary treatments in F1 (
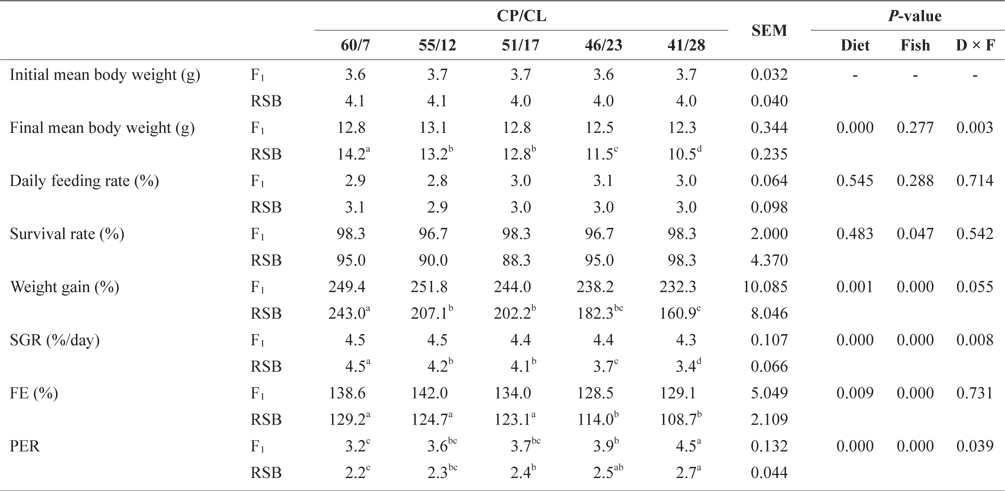
At the end of Trial 2, the experimental diets had significant effects on final mean body weight, weight gain, SGR, FE, and PER for both species (two-way ANOVA,
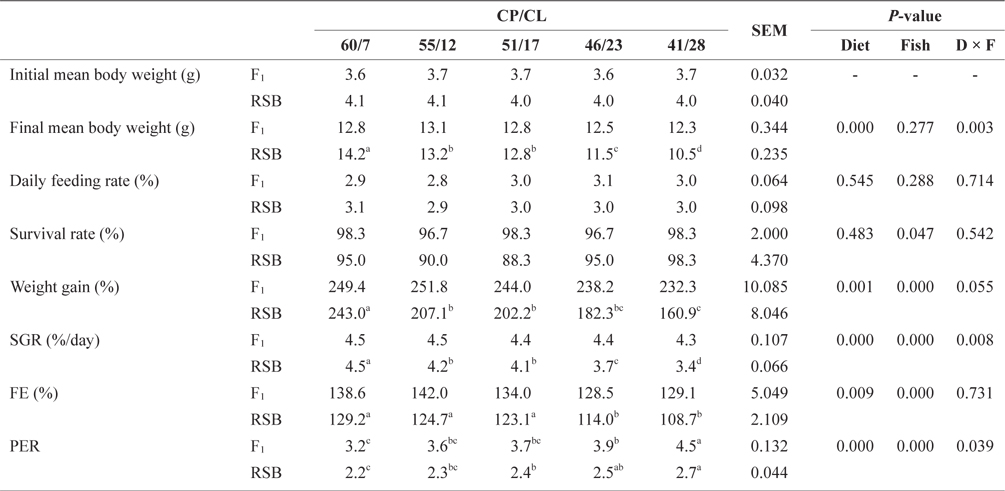
Effect of feeding diets with different protein/lipid levels on the growth performance of juvenile F1 and RSB (Trial 2)*
There was no significant difference in final mean body weight, daily feeding rate, survival, weight gain, SGR, or FE among the dietary treatments in F1. In contrast to Trial 1, the PER of F1 showed an increasing trend with decreasing dietary CP/CL. The final mean body weight, weight gain, and SGR of RSB were significantly higher on the 60/7 diet than the other diets (
The final whole body proximate compositions, except ash content, were significantly affected by dietary treatments in both species (two-way ANOVA,
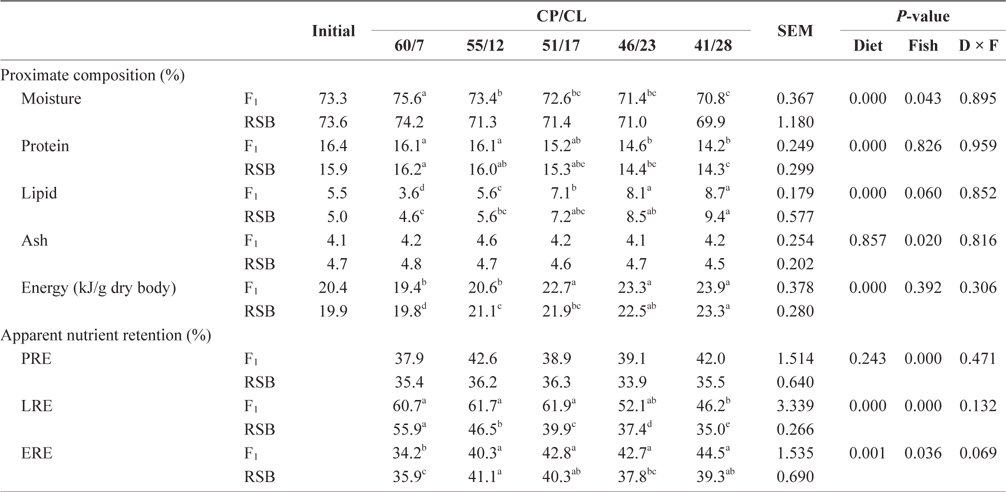
Carcass compositions and apparent protein, lipid and energy retention of juvenile F1 and RSB fed experimental diets with different protein/lipid level (Trial 2)*
With decreasing dietary CP/CL, whole body protein showed decreasing trends, but lipid and energy showed increasing trends in F1 and RSB. LRE also tended to decrease with decreasing CP/CL in both species. While F1 fed 60/7, 55/12, and 51/17 diets had significantly higher LRE than the 41/28 diet (
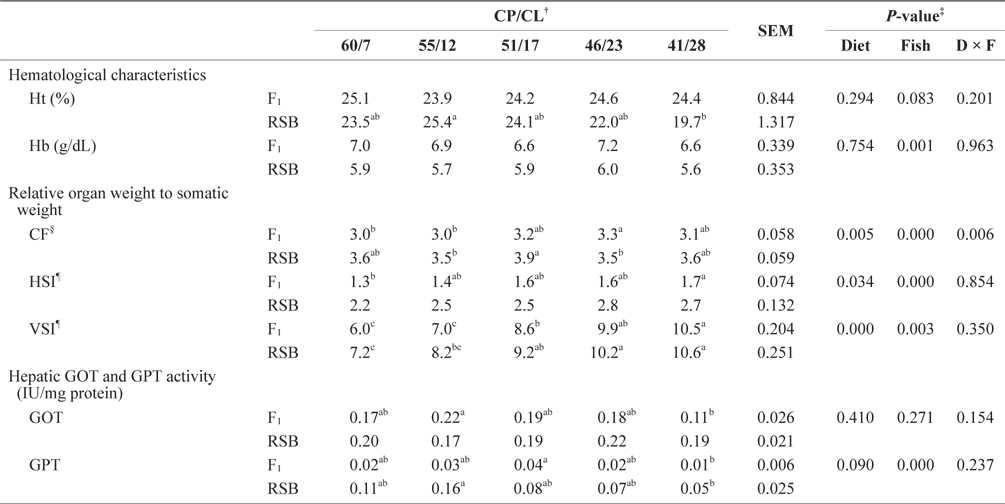
Regarding hematological parameters, only Hb contents were affected by fish species, and RSB tended to be lower than F1 (two-way ANOVA,
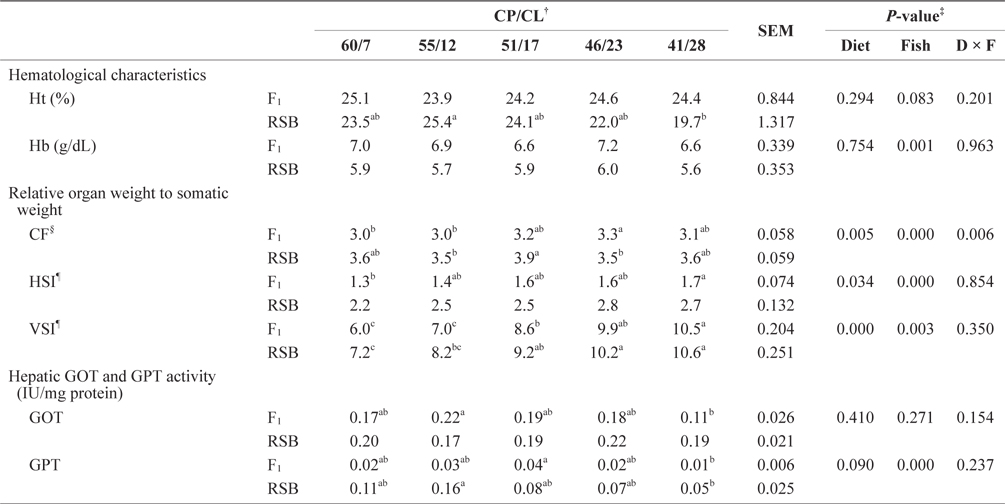
Hematological characteristics, relative organ weight to somatic weight (%), and hepatopancreatic GOT and GPT activity of juvenile F1 and RSB fed experimental diets with different protein/lipid level (Trial 2)*
The establishment of mass juvenile production for finfish aquaculture industry requires various technical disciplines and basic knowledge related to the biological, chemical, physiological, and environmental aspects of each aquaculture fish. In particular, understanding the relationship between changes in nutritional requirements and biochemical metabolism in the early life stages of fish will produce rapid improvement and success in mass fingerling production. Thus, this study was designed to identify a suitable dietary protein and lipid content and/or ratio for early F1 juveniles, which are expected to be a promising new aquaculture species in Korea and eastern China where the water temperature becomes very low in the winter.
It is well-known that the survival of fingerling production depends on whether the dietary needs for early juvenile stages are met (National Research Council, 1993; Takeuchi, 2001). In both rearing trials, survival rates were significantly different between species, with markedly higher values in F1 than RSB (
There are few reports dealing with suitable dietary protein and lipid levels in early juvenile stages of F1 or RSB. We were obligated to end the rearing Trials 1 and 2 at 2 and 4 weeks after the start due to the juveniles’ fast growth, and the resulting over-density in the rearing tanks. However, the final mean body weights in each trial increased by 2- to 3-fold relative to the initial mean body weight within this rearing period. Thus, it is reasonable to conclude that the 2- to 4-weeks rearing period for the early juvenile stages of these species are not too short to obtain reliable data.
The growth performance data revealed that suitable CP/ CL proportions for F1 and RSB were 55/12 and above 60/7, respectively, in Trial 1, whereas they were lower, at 41/28 for F1 and above 51/17 for RSB, in Trial 2. These results for RSB are consistent with the results of other authors (Yone et al., 1974; Takeuchi et al., 1991). Takeuchi et al. (1991) and Yone et al. (1974) reported that RSB weighing 1.6-29.0 g required 52-55% dietary protein and 15% dietary lipid. Watanabe et al. (1984) showed that mature RSB, weighing 689 g, required 45% dietary protein, which is markedly lower than juveniles (Takeuchi et al., 1991). In contrast, Arakawa et al. (1980) indicated a suitable dietary protein level of 40% for BSB weighing 2.9 g, which has been reported to be lower than RSB. A two-way ANOVA revealed significant differences in growth performance and nutrient retention between the two fish species. Generally, F1 performed better on diets with low CP/CL (46/23, 41/28) than did RSB, providing evidence that F1 had a lower protein requirement than the female parental fish (RSB). These findings are consistent with those in several other species where hybrid fish ordinarily showed lower protein requirements than the maternal fish (Millikin, 1982, 1983; Berger and Halver, 1987; Shiau and Huang, 1989; Tuncer et al., 1990; Brown et al., 1992; Swann et al., 1994; Brecka et al., 1995; Twibell and Brown, 1998).
Azevedo et al. (2004) found a significant difference in growth between rainbow trout and Atlantic salmon by feeding various CP/CL diets. Regarding the cause of the difference, they considered that it was due to the difference in nitrogen and energy retention efficiency between the two species. Differences in capabilities between fish species were also identified in this experiment, and effects in growth improvement by substituting fish meal for fish oil were observed in both juvenile F1 and RSB. However, significant differences (
From the hepatic transaminase activity, used as an indicator of the breakdown and consumption of amino acids, the results showed a reduced tendency in GPT activity in both species with a reduced dietary CP/CL ratio. However, there was a significant difference between F1 and RSB, with lower activity in F1 than RSB. It has been reported that feeding a high-lipid diet to fish decreases levels of glycogen, blood nitrogenous compounds, gluconeogenesis, and glycolysis enzymes (
In conclusion, these results demonstrate that the hybrid F1 is a promising aquaculture species because of its superior growth performance and higher survival rate even at a lower dietary CP/CL ratio. The results also indicated that mass juvenile production of F1 will be more economical than that of RSB.