The Scolopendrid Subspinipes Mutilans are dried centipedes which belong to the family scolopendrida [1]. The centipedes are so named because the shape of the scolopendrid resembles the human vertebra. Scolopendrid Subspinipes Mutilans, ground and mixed with alcohol, is taken as a home remedy to reduce the pain of joint diseases [2]. The scolopendrid was first mentioned in Sinnongbonchogyeong, a text in the Compendium of Material Medica that was used to treat infantile convulsion, erysipelas, kerion, scrofulosis and biting injuries [3, 4].
Pharmacopuncture treatment is a therapy that combines acupuncture with pharmacotherapy. As a single procedure, it can produce both the effects of acupuncture and herbal medicine. Moreover, a synergistic effect would be expected from this treatment because pharmacopuncture treatment does not pass through the digestive system; rather, it works fast and can be used with orally-administered medications. It is also an effective treatment for unconscious patients who are unable take medicine [5].
Scolopendrid pharmacopuncture is a complex therapy based on chemical stimulation using the biochemical pharmacological action of the scolopendrid and the physical stimulus at a meridian point. It has been used effectively for the treatment of joint pain and nerve entrapment syndrome [6, 7].
Although Scolopendrid pharmacopuncture is widely used nowadays, objective single-dose toxicity testing of it has not been conducted yet. The current research trend for single-dose toxicity testing of extracts is to study acute and subacute toxicity through the procedure of Good Laboratory Practice (GLP). All the experiments for this research were conducted at the Korea Testing & Research Institute (KTR), an institution authorized to perform non-clinical studies, under the GLP.
This study was performed to analyze the single-dose toxicity and the approximate lethal dose of the scolopendrid pharmacopuncture in rats.
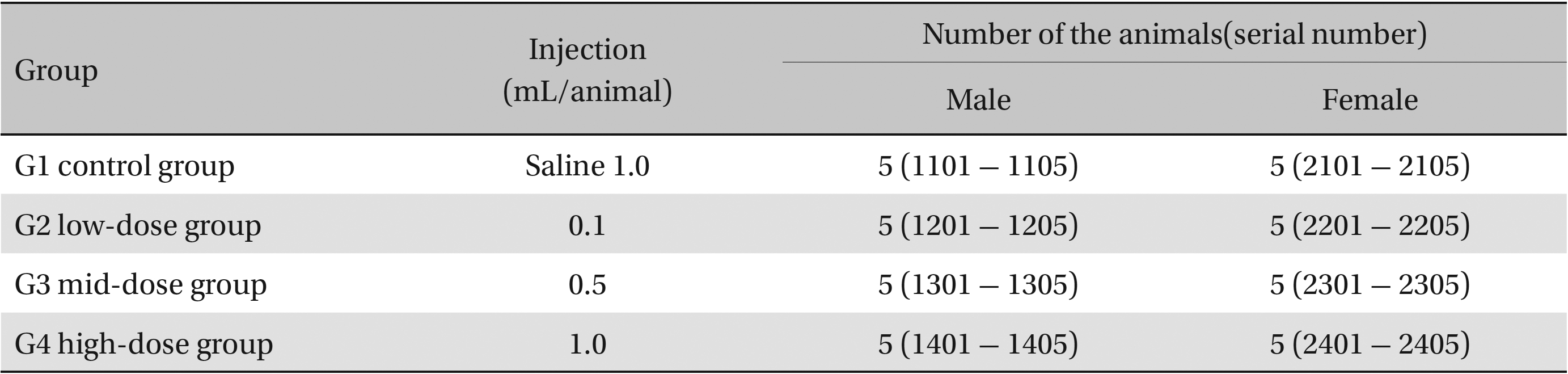
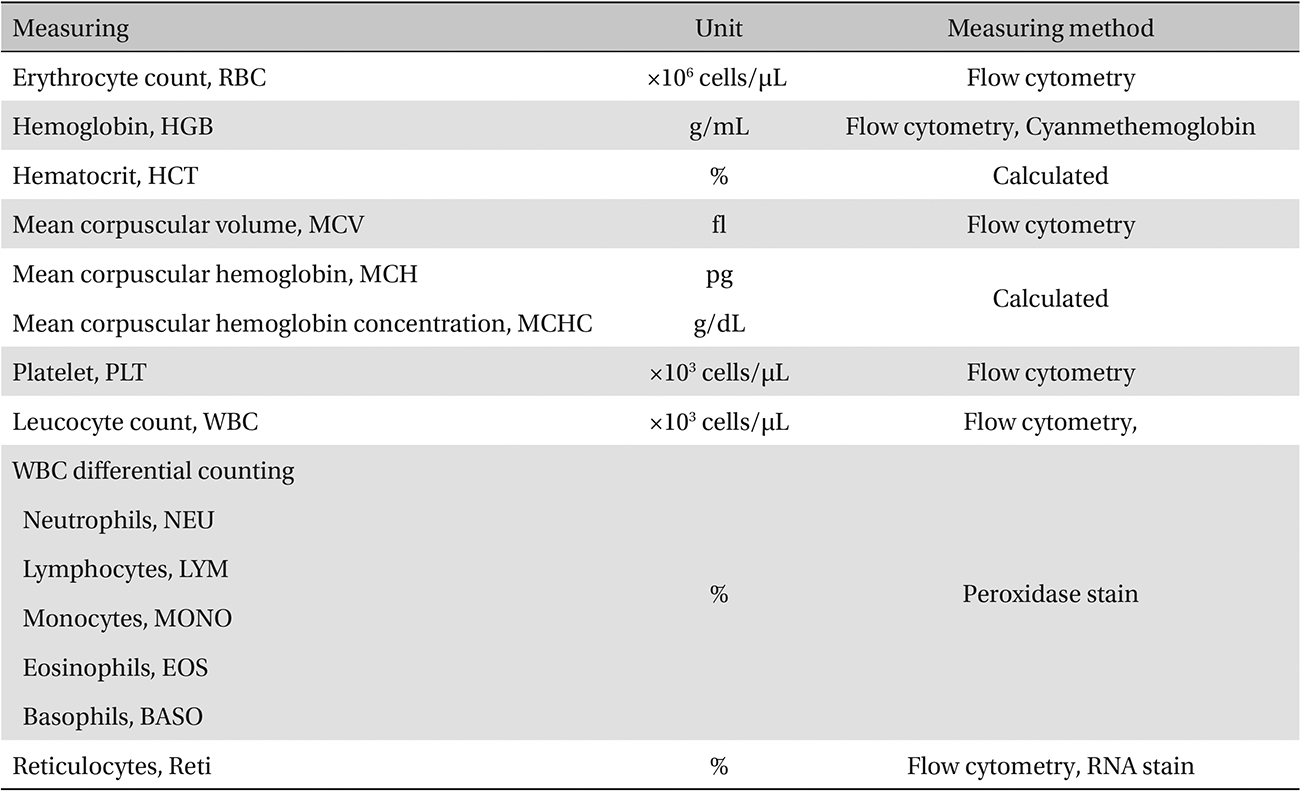
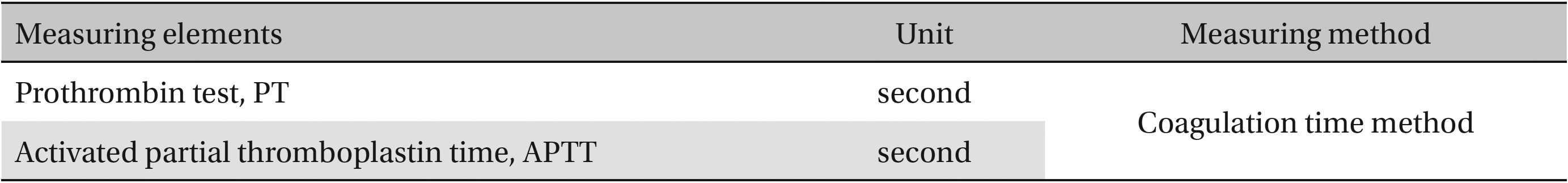
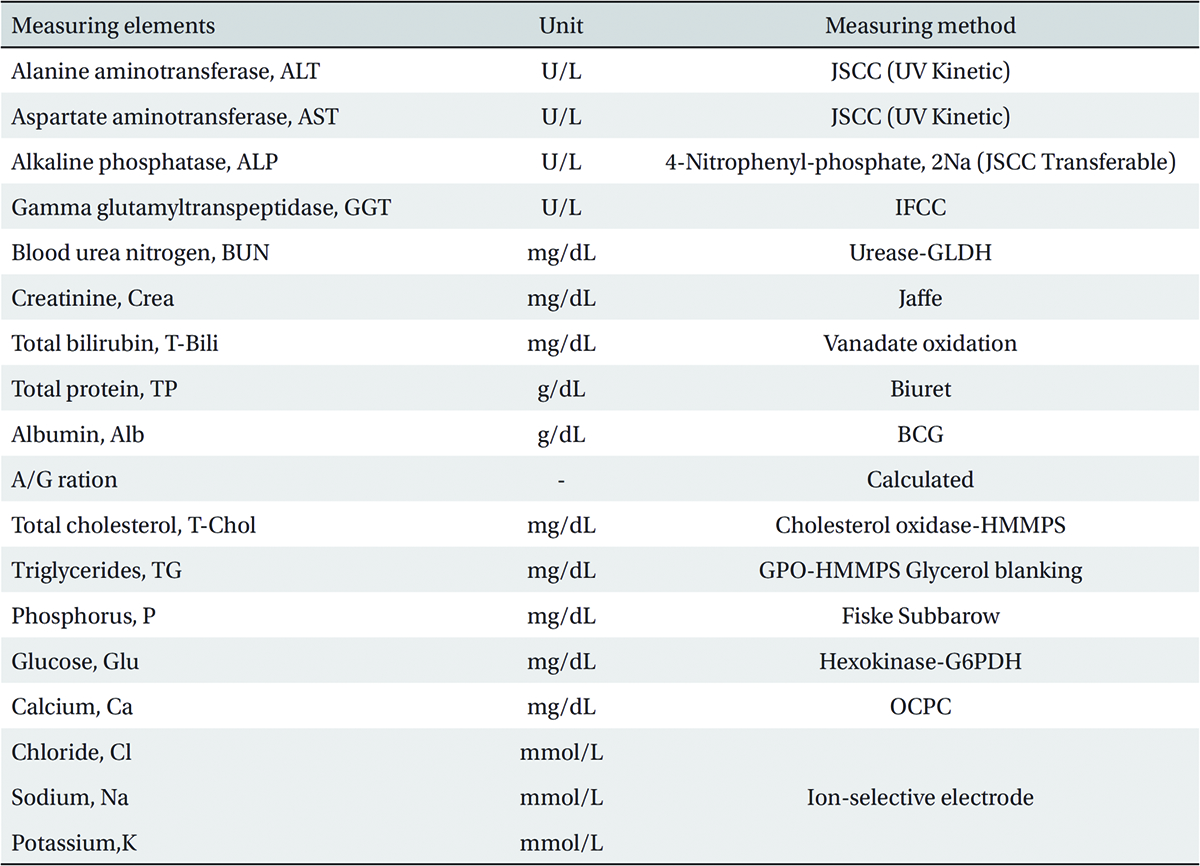
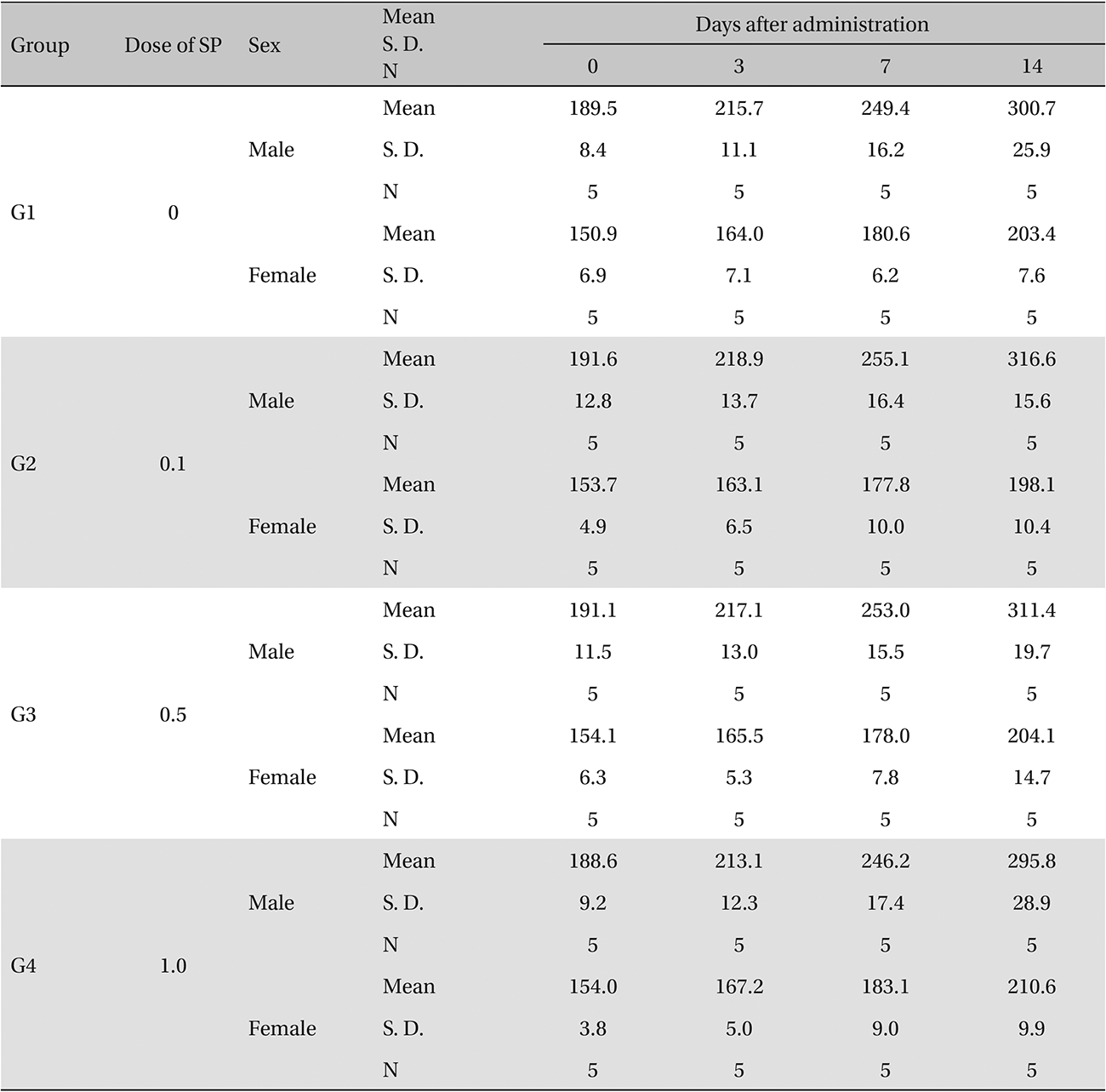
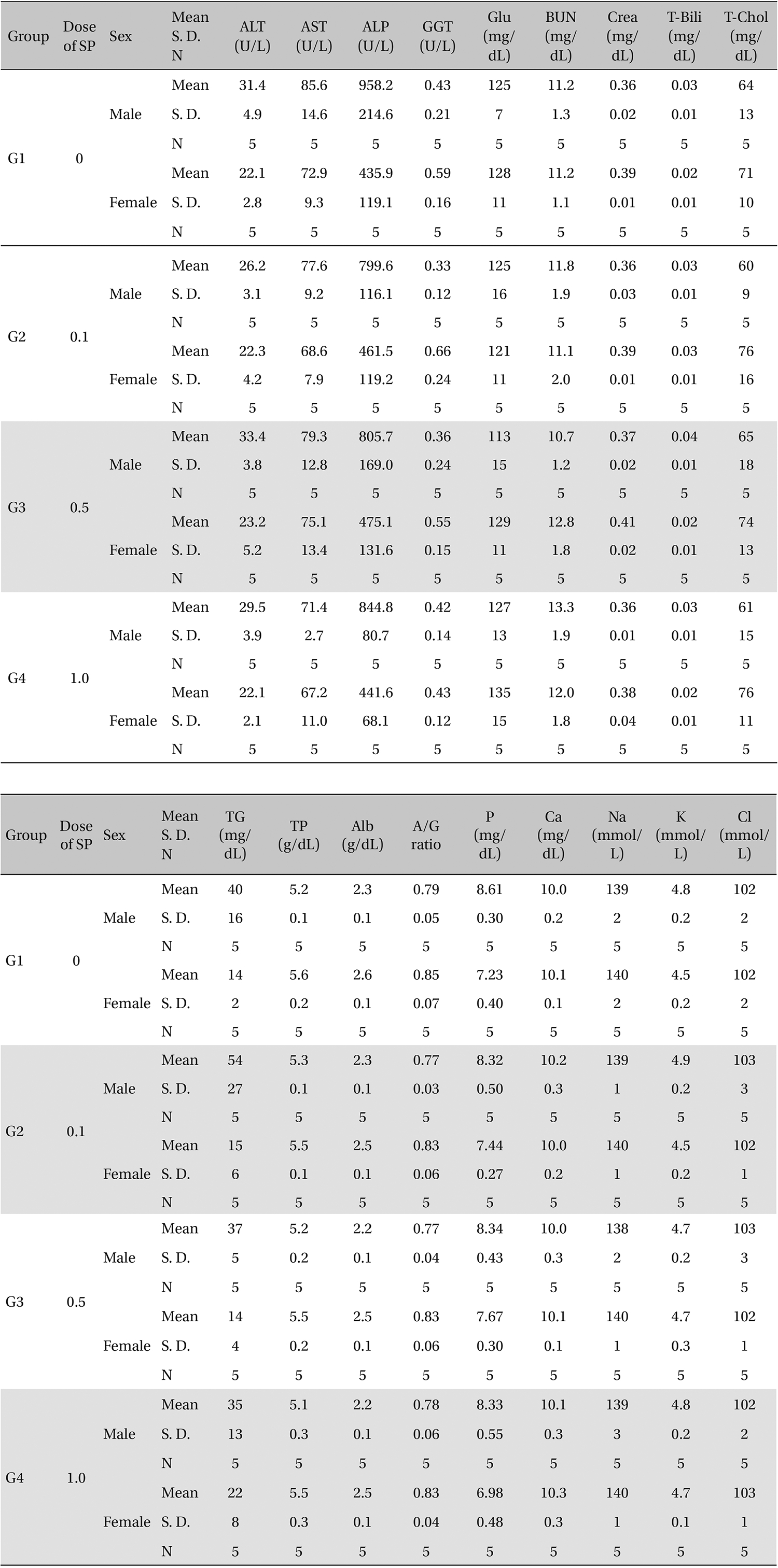
The scolopendrid pharmacopuncture was prepared in a clean room (K-GMP) in a lab at the Korean Pharmacopuncture Institute. After the mixing process with pure water, the pH was controlled to between 7.0 - 7.5. NaCl was added to make a 0.9% isotonic solution. The completed extract was stored in a refrigerator (2.1 - 6.3℃). The animals used in this study were 6-week-old Sprague-Dawley rats. The reason Sprague-Dawley rats were chosen is that they have been widely used in stability tests of medicine, so the data obtained in this study should be easily compared with many other databases. The weights of the rats were 173.4 - 208.8 g for the males and 143.0 - 161.3 g for the females at the time of injection. All animals were visually inspected and weighed using the CP3202S system (Sartorius, Germany). During 6 days of acclimatization, the rats’ general symptoms and changes in those symptoms were observed once a day. The weights were recorded on the last day of acclimatization. No abnormalities were found. The temperature of the lab was 21.0 - 23.1℃, and the humidity was 45.7% - 61.5%. Sufficient food (Teklad Certified Irradiated Global 18% Protein Rodent Diet 2918C) and UV- filtered water were provided. Groupings were done after the 6 days of acclimatization. The animals were selected by the criteria of their being close to the mean weight. In total, 20 male rats and 20 female rats were selected. The animals were distributed into 4 groups (5 mice per group, (Table 1) The expected dose of scolopendrid pharmacopuncture for clinical applications is 1.0 mL. In a pilot study, 1.0 mL/animal of scolopendrid pharmacopuncture was injected into each male and female rat; no deaths were observed. Thus, 1.0 mL/animal was set as a high dose, and 0.5 mL/animal and 0.1 mL/animal were set as a mid-dose and as a low dose, respectively. In the control group, the same doses of normal saline solution were administered. The low- and mid-dose groups of rats were injected with a single dose in the left thigh muscle, and the control group and high-dose rats group were injected with 0.5 mL/site on both thigh muscles by using disposable syringes. This study was conducted under the approval of the Institutional Animal Ethics Committee. On the day of dosing (day 0), the general symptoms (the type of toxic symptoms, revealing time, recovering time, etc.) and the mortality were examined after 30 minutes, 1 hour, and 2, 4, and 6 hours. From the 1st day to the 14th day of treatment, the general symptoms were examined once a day. The weight was measured immediately before treatment, and at 3, 7 and 14 days after treatment. After the rats have fasted for more than 18 hours before the necropsy, under anesthesia, blood samples were taken from the abdominal aorta (15 days after injection). Hematologic examination results were obtained by using an automatic hematology analyzer (ADVIA 120, SIEMEMS, Germany) to analyze the blood samples (Table 2). Two-mL blood samples were centrifuged for the blood coagulation test (3,000 rpm, 10 minutes). Coagulation test results were measured by using an automated coagulation analyzer (Coapresta 2000, SEKISUI, Japan) (Table 3). In addition to blood hematology, blood taken from the abdominal aorta was used in blood chemistry tests. Blood chemical test results were obtained using an automatic analyzer (7180, HITACHI, Japan) and electrolyte analyzer (AVL9181, Roche, Germany) (Table 4). After the termination of observation, all surviving animal organs and tissues were visually inspected and microscopically examined. The weight, hematologic examination and blood chemistry analysis results from the experiment were analyzed by SAS software (version 9.2, 9.3, SAS Institute Inc., USA). The Bartlett test was conducted to evaluate the homogeneity of variance and the significance. The one-way ANOVA test was conducted when homogeneity of variance was recognized, and the Kruskal-Wallis test was conducted posthoc.
Groups of animals
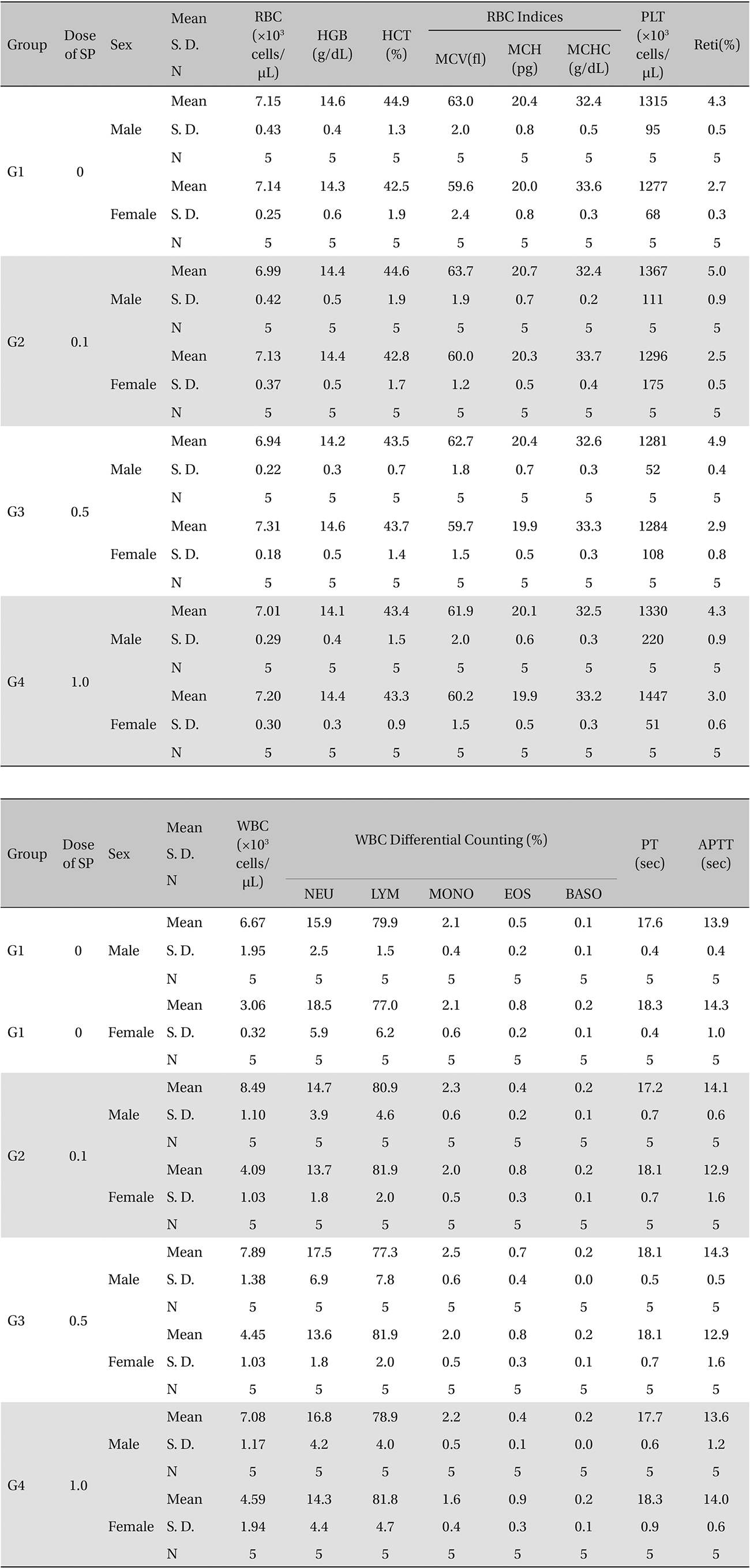
[Table. 2] Hematologic examination
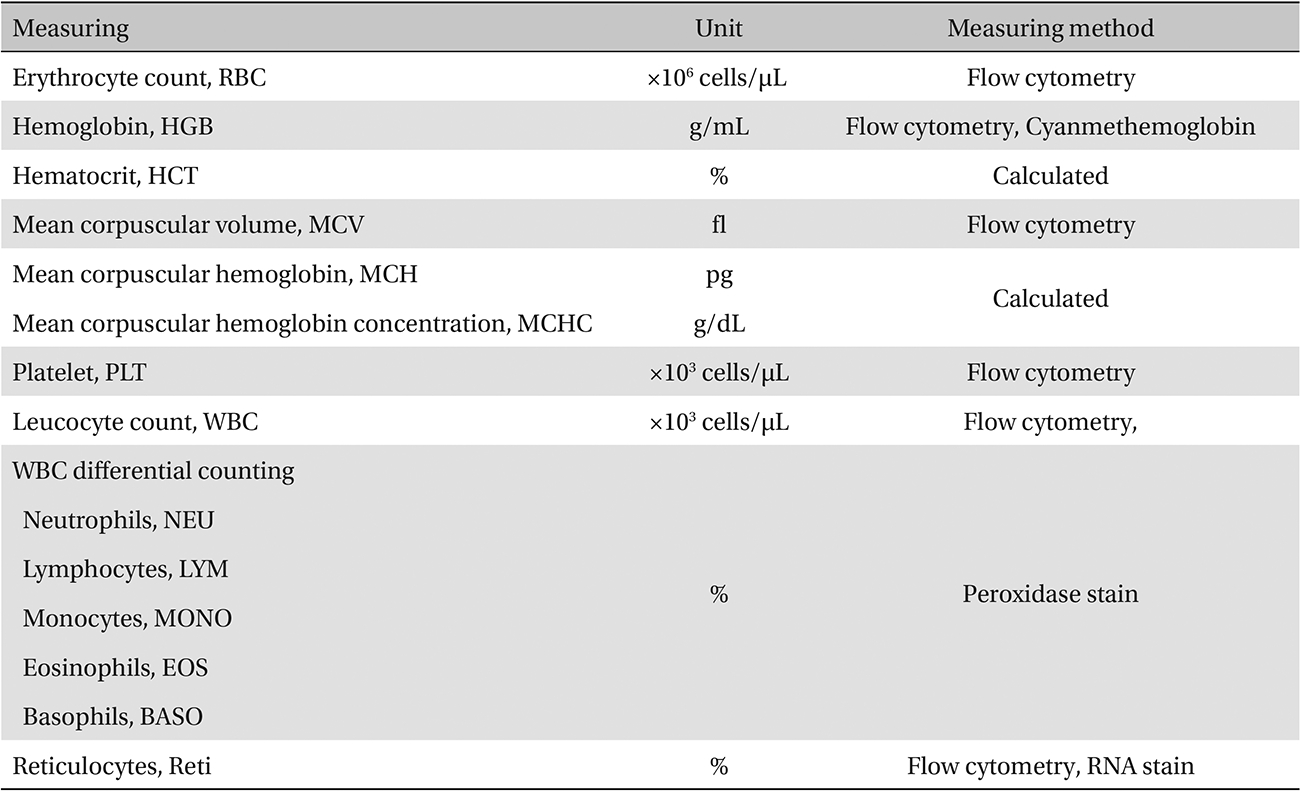
Hematologic examination
Coagulation test
[Table. 4] Blood chemical test
Blood chemical test
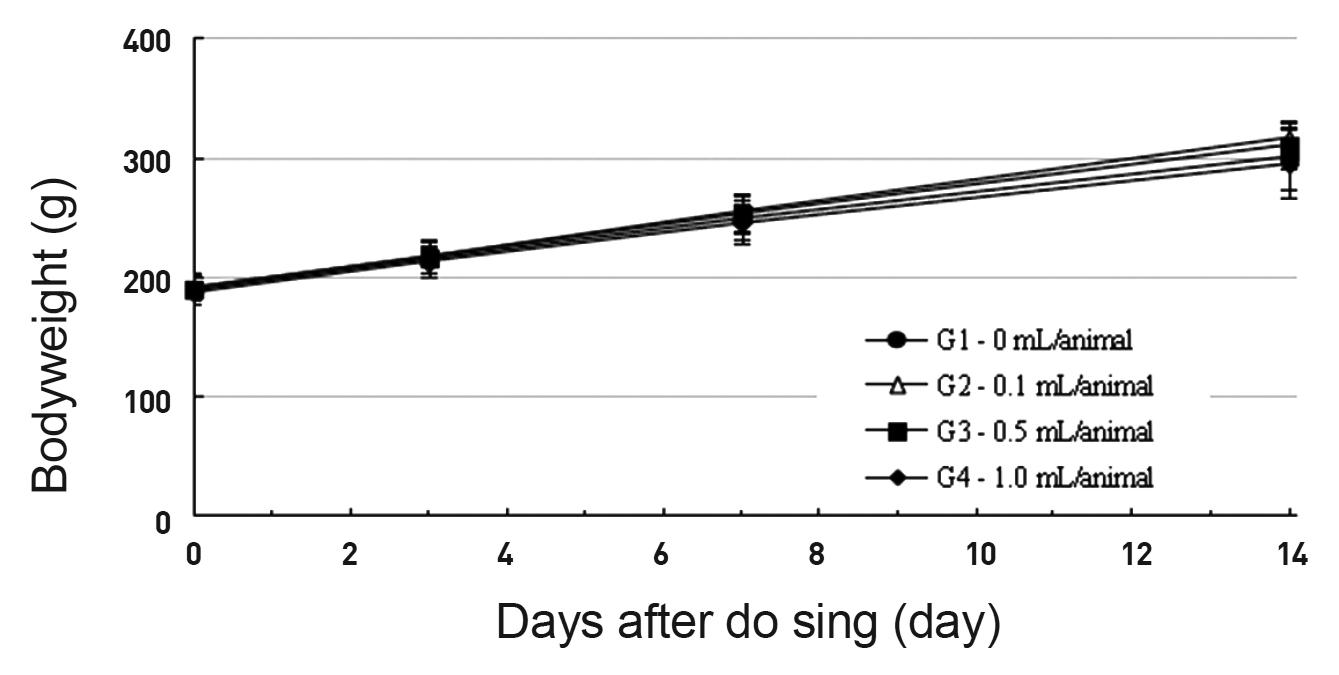
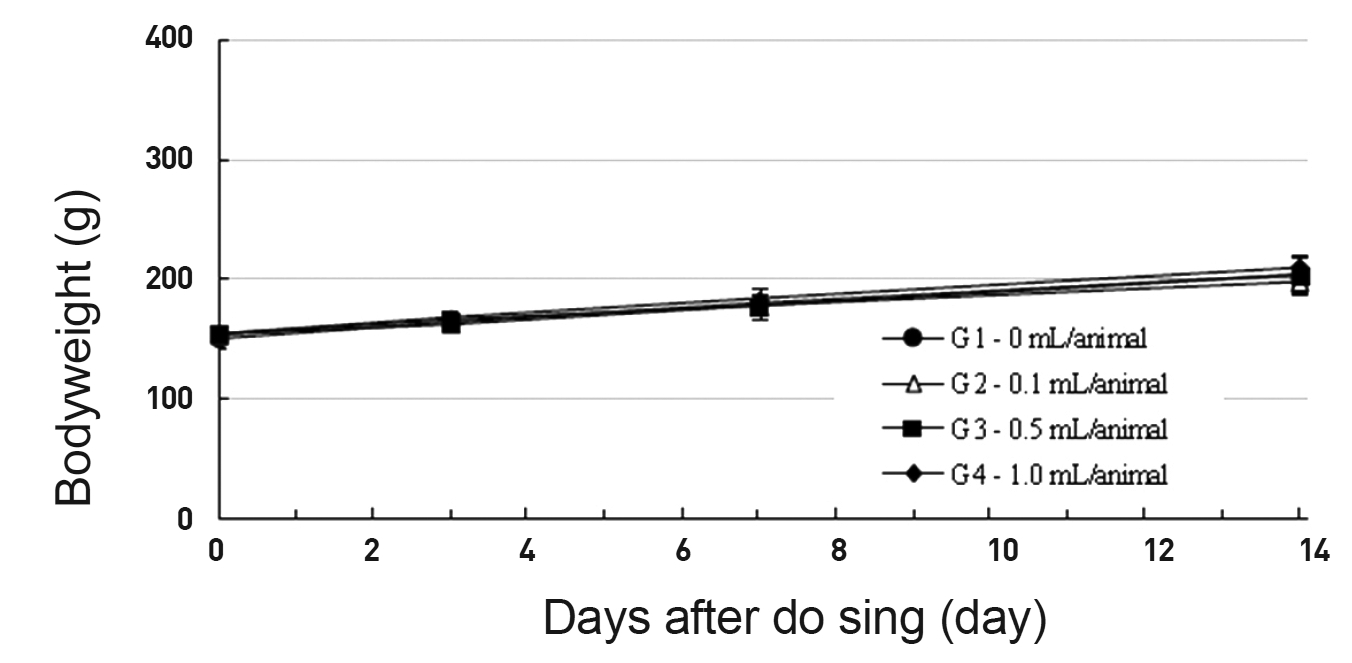
In this study, no deaths or abnormal symptoms occurred. This was the same in all groups. Also, no changes in weight were observed in any of the groups. Evaluations were carried out every day, and on the 3rd, 7th and 14th days after administration (Table 5 , Fig 1 and 2). The results of hematologic examinations and blood chemistry analyses are shown in Table 6 and 7, respectively. No meaningful changes were noted on necropsy, and after histological examination of all of the groups, no significant changes related to injections in the brain, lungs, liver, kidney or spinal cord were noted.
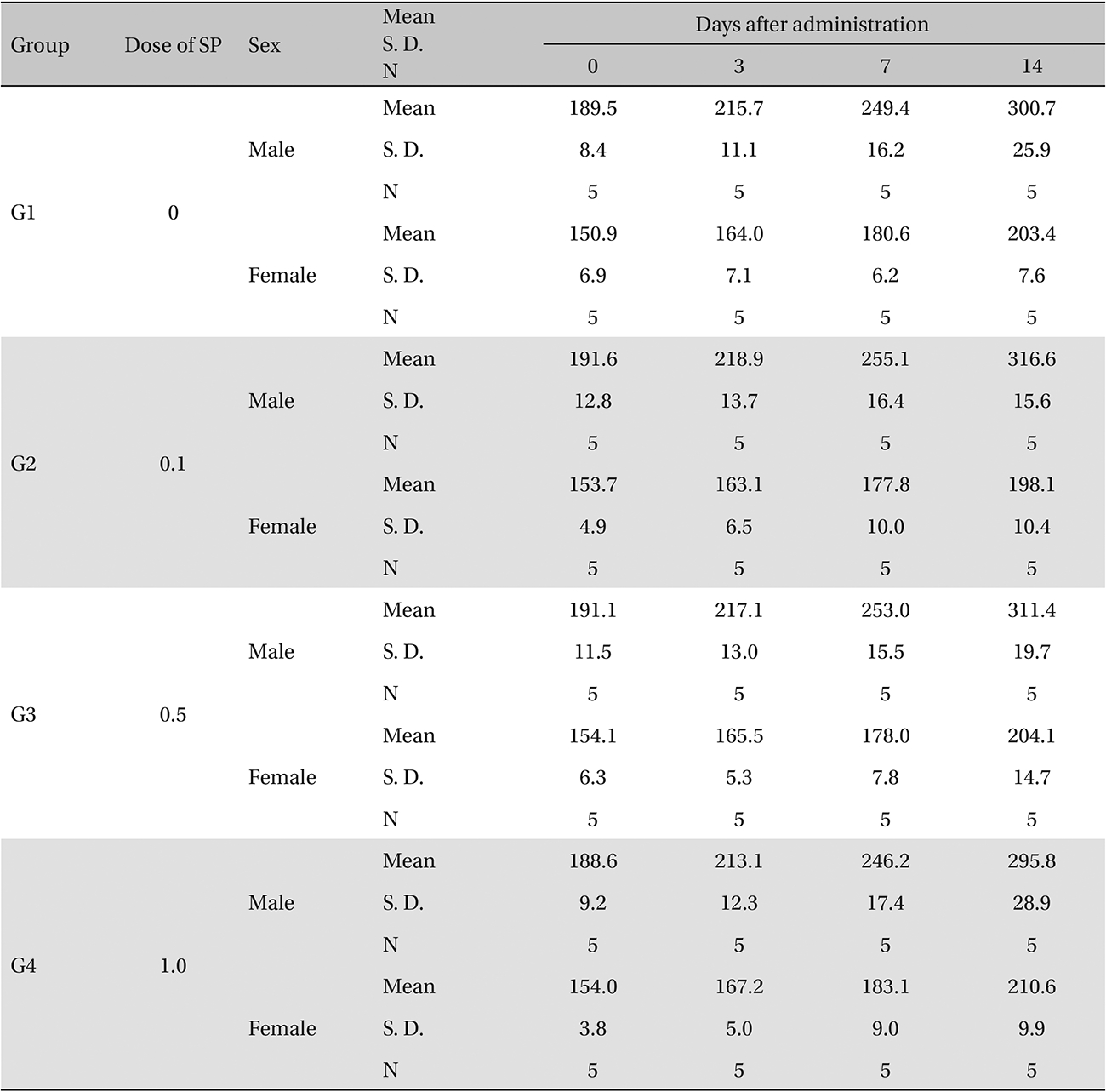
Body weights (g)
[Table. 6] Mean Hematology Parameters
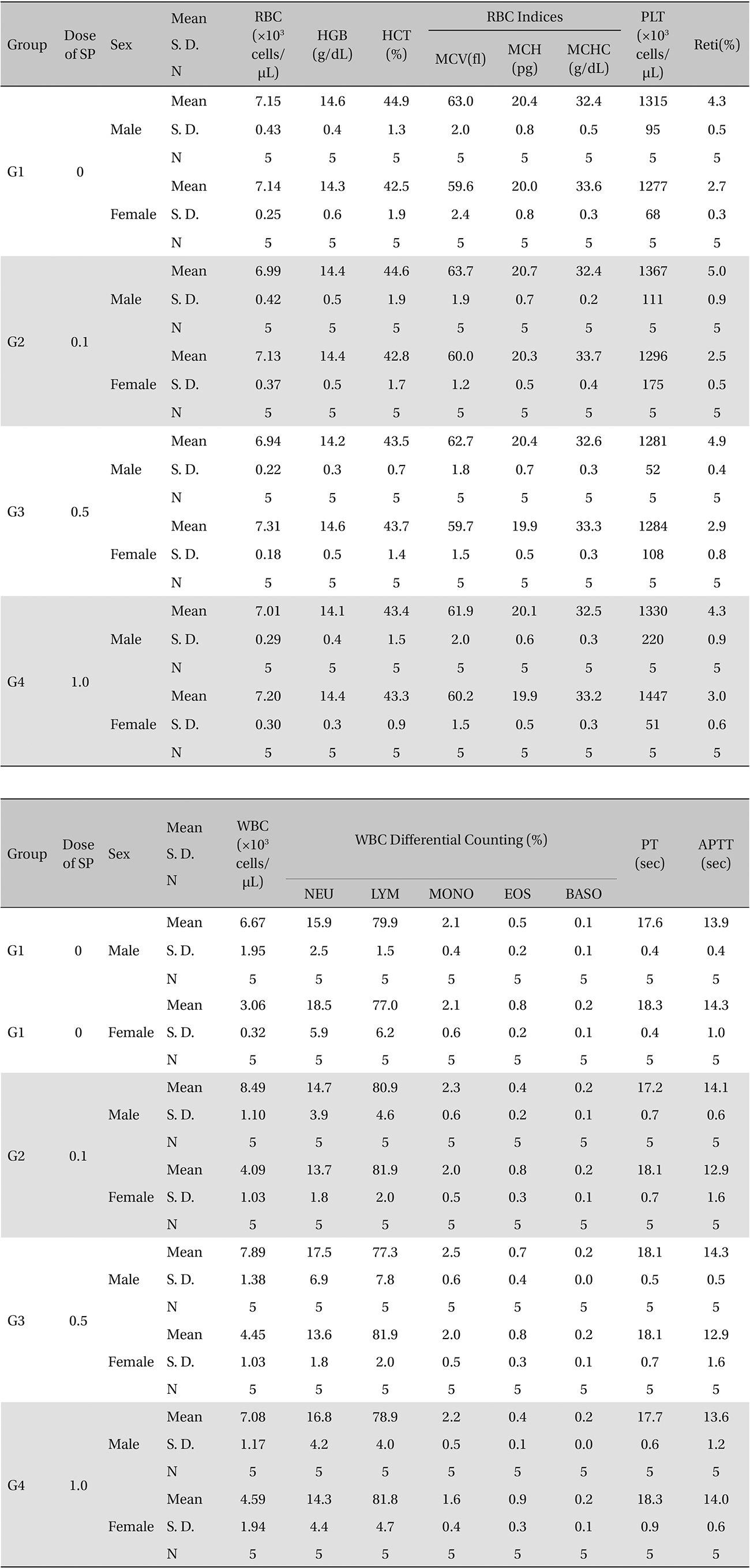
Mean Hematology Parameters
[Table. 7] Mean clinical chemistry
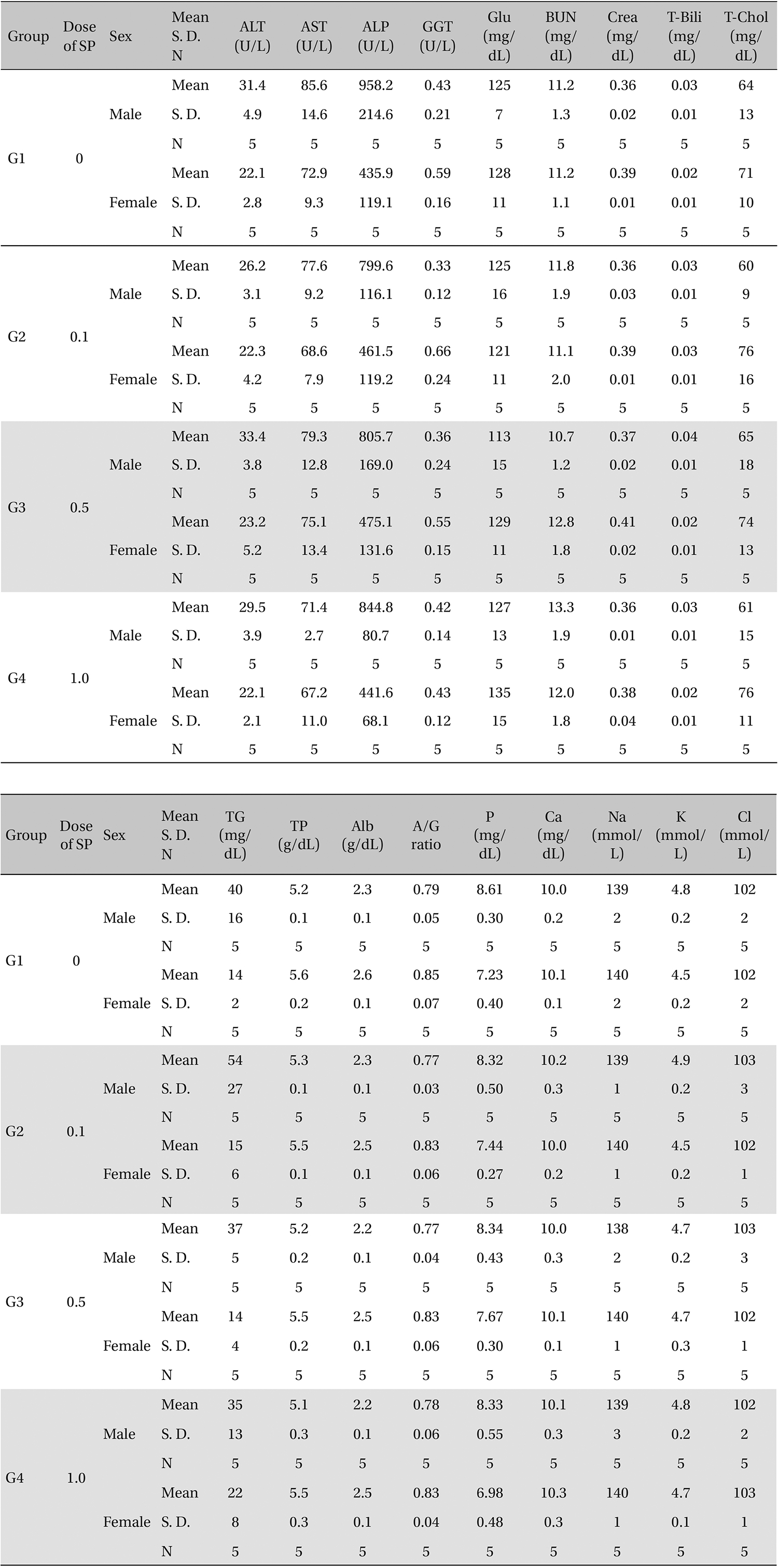
Mean clinical chemistry
Much experimental research on and clinical trials of scolopendrid pharmacopuncture have been done. Hong reported that scolopendrid has the effects of reducing pains and alleviating fever and relaxing smooth muscles [8]. Kim reported antipyretic, analgesic and anticonvulsive effects of scolopendrid. The effects of scolopendrid have been reported to be greater when the head and the legs are removed [9]. In a safety study of scolopendrid pharmacopuncture, Lim
This study was performed to prove more objectively the safety of scolopendrid pharmacopuncture than previously recorded. Sprague-Dawley rats were chosen for this study. Scolopendrid pharmacopuncture, 0.1-mL, 0.5-mL, and 1.0-mL doses, was administered to the experimental group, and normal saline solution, a 1.0-mL dose, was administered to the control group. No significant differences in the clinical signs, weights, and results of hematological examinations or blood chemistry analyses were observed between the control group and the experimental group. The necropsy for checking for abnormalities in organs and tissues showed no significant histological findings. To assess the toxicity of scolopendrid pharmacopuncture, further studies about the acute and the chronic harmful effects and the relations with the capacity reaction are necessary. Animal testing is the most fundamental and basic way to study safety assessments. For the study of toxicity, from the Korea Food & Drug Administration has a testing protocol guideline [12], and all the experiments should be conducted following GLP regulations. In this study, the LD50 of scolopendrid pharmacopuncture can be assumed to be above 1.0 mL in both male and female rats, indicating that this dose is safe to use and does not cause histological abnormalities.
The objective of this study was to analyze the single-dose toxicity of scolopendrid pharmacopuncture. All experiments were conducted at the KTR, an institution authorized to perform non-clinical studies, under the regulations of GLP. Results show that the administration of 1.0 mL/animal of scolopendrid pharmacopuncture does not cause any changes in weight or any mortality. This would indicate that this dosage of scolopendrid pharmacopuncture can be safely used for treatment.