The risk of cancer is now a burning issue throughout the world [1]. According to the World Health organization (WHO, 2010), lung cancer is the most commonOriginally diagnosed cancer with 1.4 million new cases reported every year [2, 3]. Non small cell lung cancer (NSCLC) is more prevalent, accounting for nearly 80% of all lung cancer cases [4]. Tobacco smoking is a well established factor for lung carcinogenesis; a nearly ten-fold increase in NSCLC is attributed to long-term cigarette-smoking [5]. Benzo[a]pyrene (BaP) is a common polycyclic aromatic hydrocarbon found in cigarette smoke and is responsible for inducing lung cancer. During carcinogenesis, BaP is metabolized to (±)-B[a]P-r-7,t-8-dihydrodiol-t-9,10-epoxide (BPDE) which causes lung carcinogenesis [6].
Surgical resection is the only treatment for NSCLC patients, and patients with advanced stages are treated with a combination of surgery, chemotherapy and radiation therapy [7]. In an orthodox regimen, advanced NSCLC is often treated with cisplatin or carboplatin, in combination with gemcitabine, paclitaxel or docetaxel, which have significant side-effects on normal cells [8]. In view of undesirable side-effects, a search is on to find ways to avoid these by using some alternative agents and/or drugs that are equally effective, but have few or no side-effects.
Natural medicines, including herbal and plant products, have been widely used in modern therapeutic applications to treat different human diseases, including cancer [9]. All these traditional medicines are included in complementary and alternative medicines (CAM). The main advantage of CAM therapies is low cost with fewer side effects [10]. Thus, extracts from traditional medicinal plants and their active components have re-ignited research to ascertain and evaluate their scientific roles in cancer treatment. As one of the hallmarks of cancer is the immortality of cells that do not have to depend on a supply of nutrients to proliferate, an important landmark of all anti-cancer drugs is their ability to induce apoptosis in these obstinate cells [11].
In the search for different alternative drugs for use against lung cancer (NSCLC), ethanolic extract of
Three (3) to 4 months old healthy white rats (
A randomized set of 85 rats were used for each time point and were again sub-divided into 7 different groups consisting of 5 rats each as follows:
The experimental data were collected 1 (5th month), 2 (6th month) and 3 (7th month) months after the administration of the drugs had begun to assess the possible effect of Condurango at three post-cancer time points.
BaP was prepared by mixing with olive oil, and a dose of 50 mg/kg of body weight was given to each rat twice a week (Tuesday and Friday) for one month through gavage [13]. After one month of scheduled BaP feeding, rats were fed a normal diet. At the end of four months, lung cancer developed in BaP-intoxicated rats, which had earlier been confirmed in a trial experiment.
Stock solution of ethanolic extract of Condurango dissolved in 65% ethyl alcohol was prepared by mixing 1 mL of crude extract with 20 mL of distilled water [14]. To determine whether any liver toxicity was produced by Condurango, we fed normal rats one drop (0.06 mL) of Condurango from the prepared stock solution twice a day with the aid of a fine pipette [14]. The rats were allowed access to normal food and water. We observed no mortality or changes in behavior in the rats during a 24-to 48-hour observation. Therefore, this dose was selected for further indepth study.
Each BaP-induced lung-cancer-bearing rat was fed with one drop (0.06 mL) of Condurango from the stock solution or 65% ethyl alcohol (vehicle of Condurango), as the case may be, twice a day with the aid of fine pipette [14] for the next 1, 2 or 3 months after cancer had developed (post-cancer drug treatment).
Lung samples were very carefully removed and immediately washed with phosphate buffer solution (PBS) three times. Then, the lung samples were fixed with 4% formalin (preservative) and kept at room temperature for a few days (five to seven days) to keep the lung tissues intact. The formalin-fixed lung samples were dehydrated with graded alcohol (50% - 100%), twice rinsed in xylene (30 minutes each), and then transferred to the embedding bath. The tissues in paraffin blocks were cut into ribbon-like pieces of 5 μm in thickness with a microtome and were placed onto greeze-free slides after stretching in warm water. The paraffin was removed by using xylene (two washes each for 10 minutes), and the lung sections were stained with hematoxylin for 2 minutes, followed by washing in distilled and tap water, respectively. Then, the tissues were brought into an alcohol medium (70% and 90%, two washes each for 2 minutes), stained with eosin (30 seconds) and dehydrated in 90% and absolute alcohol (two washes each for 2 minutes). Xylene treatment (two changes each for 2 minutes) was carried out, and lung sections were mounted by using DPX (Dibutyl Phathalate Xylene) ith cover slips. Finally, the histological characteristics of the lung sections from the different groups of rats were photographed under a light microscope (Leica, Germany), and the data were recorded.
Lungs were quickly removed from each rat, and lung epithelial cells were flushed out gently with the aid of a hypodermic syringe. Finally, the cells were collected by centrifugation [15]. To estimate the reactive oxygen species (ROS) we incubated the perfused live lung epithelial cells with Dichloro-dihydro-fluorescein diacetate (DCFHDA) (20 μM) for 30 minutes at 37°C, and we determined the fluorescence intensity of DCFHDA by using a flowcytometer (FACS Callibur; BD Bioscience).
The lung and liver tissues (50 mg) were washed twice with 1 × PBS immediately after dissection and were homogehomogenized in 1.5 mL of protein lysis buffer (Tris-HCl, pH 8.0, 50 mM, Nonidet P-40-1%, NaCl: 125 mM, NaF: 1 mM, phenylmthylsulfonyl fluoride: 1 mM, aprotinin: 1 μg/mL, sodium orthovanadate (NaVO4): 1 mM, sodium pyrophosphate: 10 mM). The homogenized tissues were spun in a cold centrifuge (REMI C24 Model, India) at about 6000 × g for 15 minutes at 4°C. After centrifugation, the supernatants were used directly as aliquots or stored at -20°C until they were used within the next seven days (generally within a day for enzymatic studies). The total protein content was measured by using the Bradford method [16]. The activities of catalase (CAT) [17], superoxide dismutase (SOD) [18] and glutathione (GSH) [19] from lung homogenates and aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels [20] from liver homogenates were spectrophotometrically analyzed.
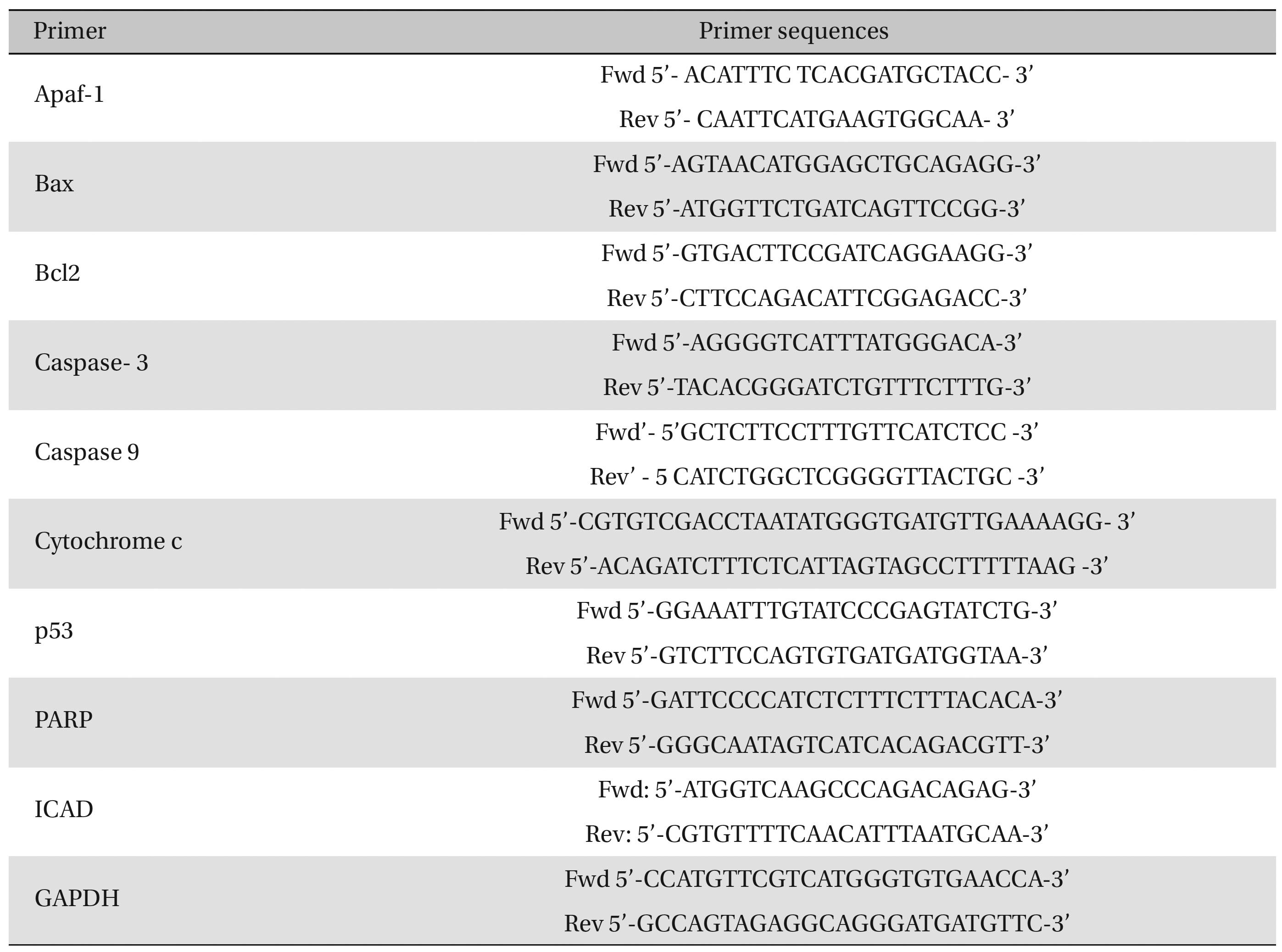
Fifty (50) mg of lung-tissue samples were lysed in 0.5-mL TRIzol reagent, homogenized for 5 minutes, and kept for 5-10 minutes at room temperature (RT). Then, the tissue homogenates were centrifuged at 9500 × g for approximately 10 minutes, and the supernatant was collected. The supernatant was incubated at RT for 5 minutes, and an equal amount of chloroform was added to the cell suspension. The mixture was kept at RT for 5 minutes and centrifuged at about 9500 × g for 15 minutes at 2 - 8°C. The aqueous phase was collected carefully and placed in a new 1.5-mL centrifuge tube. Five hundred (500) μL of cold isopropyl alcohol was added to that supernatant and incubated at 30°C for 10 minutes. The sample mixture was centrifuged at about 13500 × g for 12 minutes, and the RNA pellet was collected by discarding the supernatant. The RNA pellet was washed with 0.5 mL of cold 75% ethanol by centrifugation at about 9500 × g for 10 minutes. Then, the supernatant was discarded, and the RNA pellet was air-dried until the alcohol had been fully vaporized. Finally, the dried RNA pellet was dissolved in 50 μL of diethylpyrocarbonate (DEPC)-treated water. The diluted RNA was then heated at 65°C for 10 minutes, kept for 2 minutes at RT, and then immediately transferred to a -20°C area for storage and future use. RNA concentrations were measured by using the orcinol method [21]. Expressions of different pro- (Bax, cytochrome-c, caspase-9, caspase-3, ICAD, and PARP) and anti-apoptotic gene (Bcl-2) were analyzed by using semi-quantitative Reverse Transcriptase- Polymerase Chain Reaction (RT-PCR). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as used as a house-keeping gene. We undertook the standard protocol for the preparation of cDNA [22] and amplification with primer sequences (Table 1) Band intensities were analyzed densitometrically by using ImageJ software (Germany).
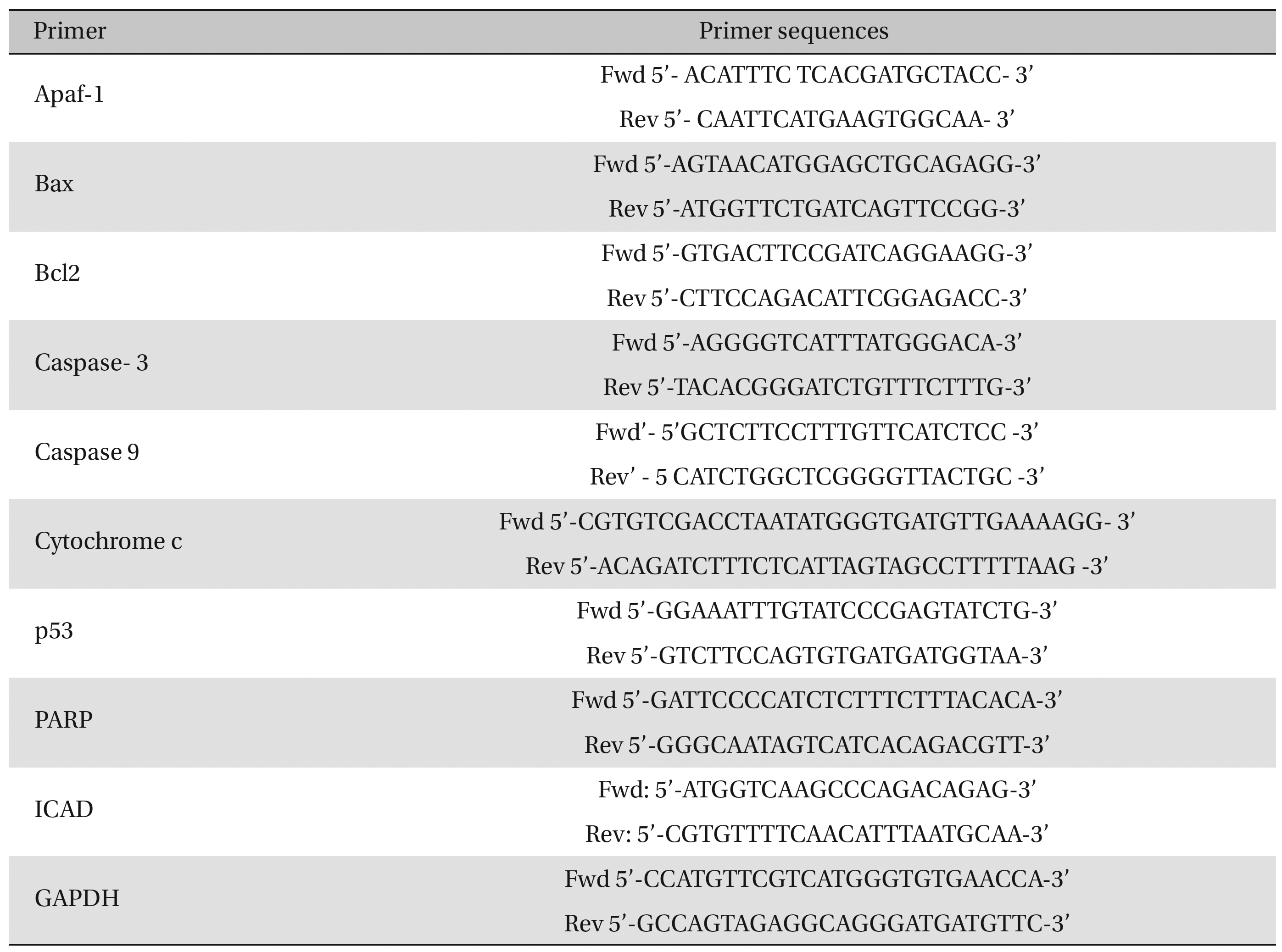
Primer sequences
The protein activities of apoptotic markers were measured by using indirect ELISA (Enzyme linked immuno-sorbent assay) with an ELISA reader. The color intensity was measured at 405 nm with respect to a blank.
Fifty (50) μg of proteins were separated from each sample by using sodium dodecyl sulfate- polyacrylamide gel electrophoresis (SDS-PAGE) and the proteins were transferred to a Polyvinylidene fluoride (PVDF) membrane in a tank blotter (Bio-Rad, USA). After protein transfer, the membranes were incubated with primary antibodies (caspase-3 and PARP) at 4°C overnight. GAPDH was used as a house-keeping control. The membranes were incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies [23]. The H2O2-3,3’-diaminobenzidine (DAB) system was used as a developer, and band intensities were analyzed densitometrically using ImageJ software (Germany).
DNA was extracted from each lung (50 mg) by using the conventional phenol-chloroform (1 : 1) method, was separated in a 2% agarose gel containing ethidium bromide, and was visualized under an UV transilluminator [12].
The observers were blinded during observation as to whether they were observing the controls or materials from the Condurango-treated animals.
Data were analyzed, and the significance of differences between the mean values was determined by using the one-way analysis of variance (ANOVA) with Fisher’s least significant difference (LSD) post-hoc tests with SPSS 14 software (SPSS Inc, Chicago, IL, USA). Statistical signifi- cance was set at
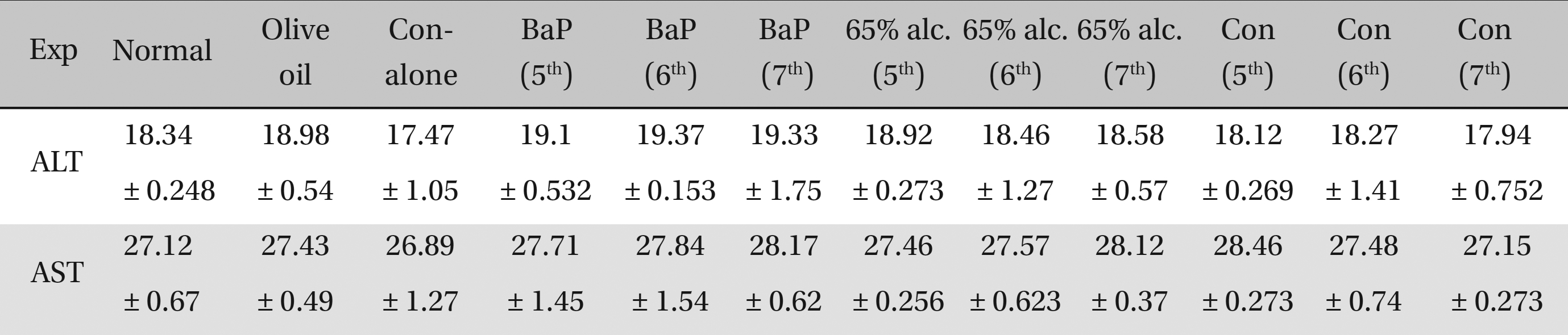
No significant differences in ALT and AST activities were observed in either the cancerous or the BaP + 65% alcohol- treated groups (Table 2). No significant differences in ALT and AST activities were observed among the normal, the olive-oil fed, the 65% alcohol-fed and the Condurango alone-treated controls. Therefore, the olive-oil-fed, the only 65% alcohol-fed and the only Condurango-fed controls were excluded from further studies, keeping only BaP-fed (cancer-bearing) and BaP + 65% alcohol-fed groups as controls.
[Table. 2] AST-ALT estimate of the effect of Condurango (Con) on rats
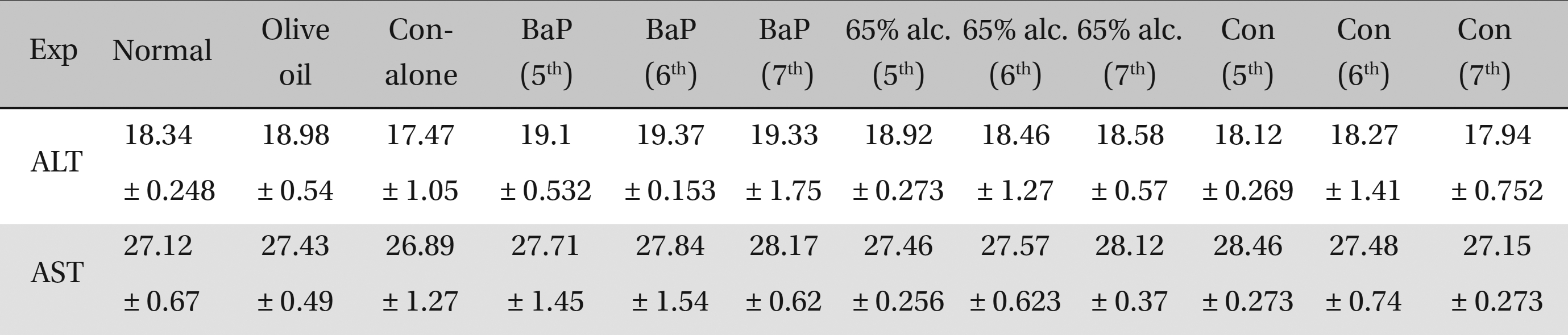
AST-ALT estimate of the effect of Condurango (Con) on rats
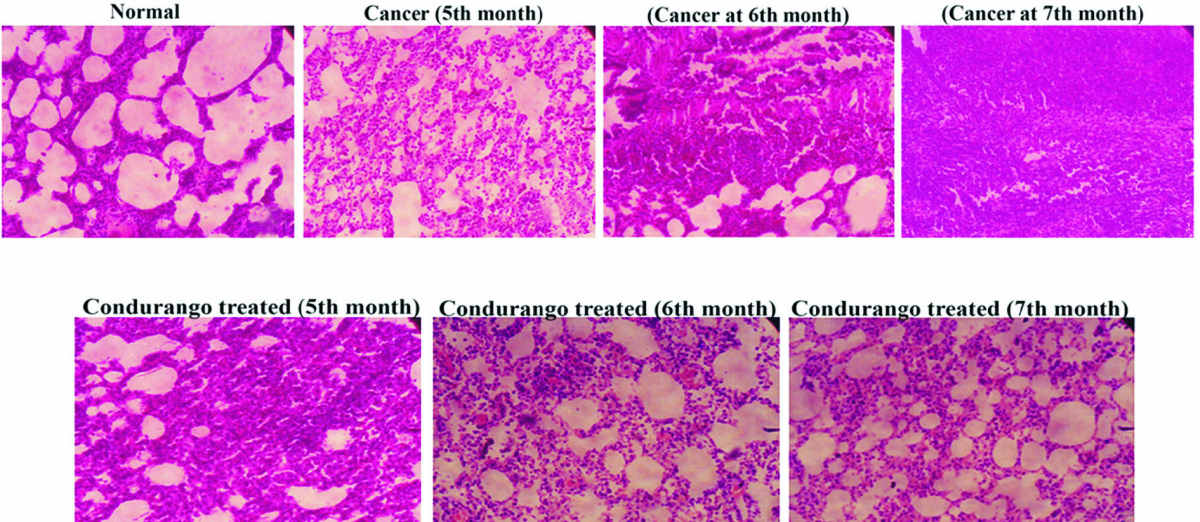
Histological analysis clearly demonstrated a uniform arrangement of lung alveolar spaces with regularly arranged small nuclei-filled cells in normal lung sections. However, the alveolar spaces were gradually condensed and became stiff, which increased with the increasing cancer development in cancerous lungs. Lung sections of Condurango- treated rats at the 5th and the 6th month did not show much recovery; after three months of drug administration, considerable recovery, with regular tissue arrangements and airy alveolar spaces, was noted (Fig 1).
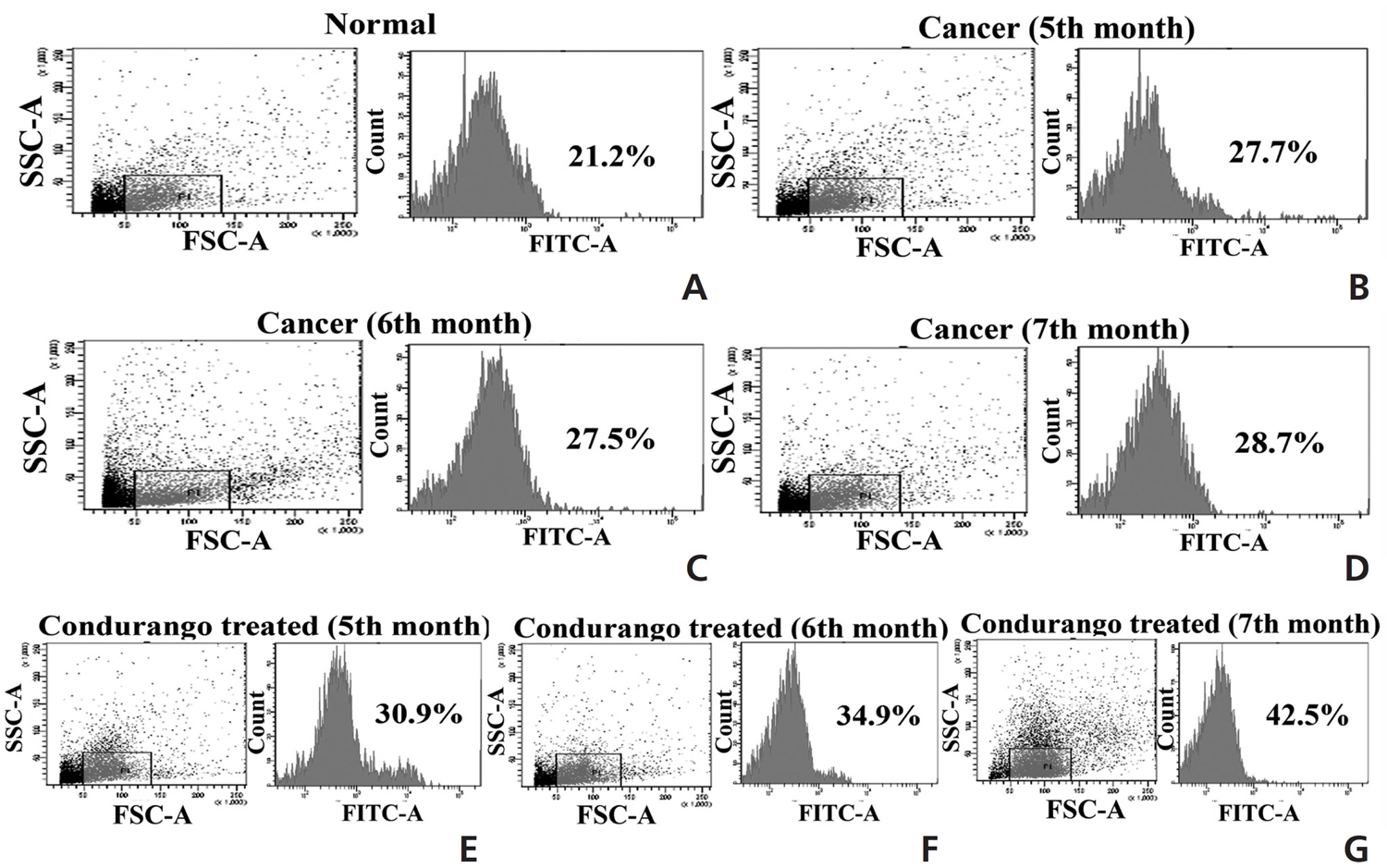
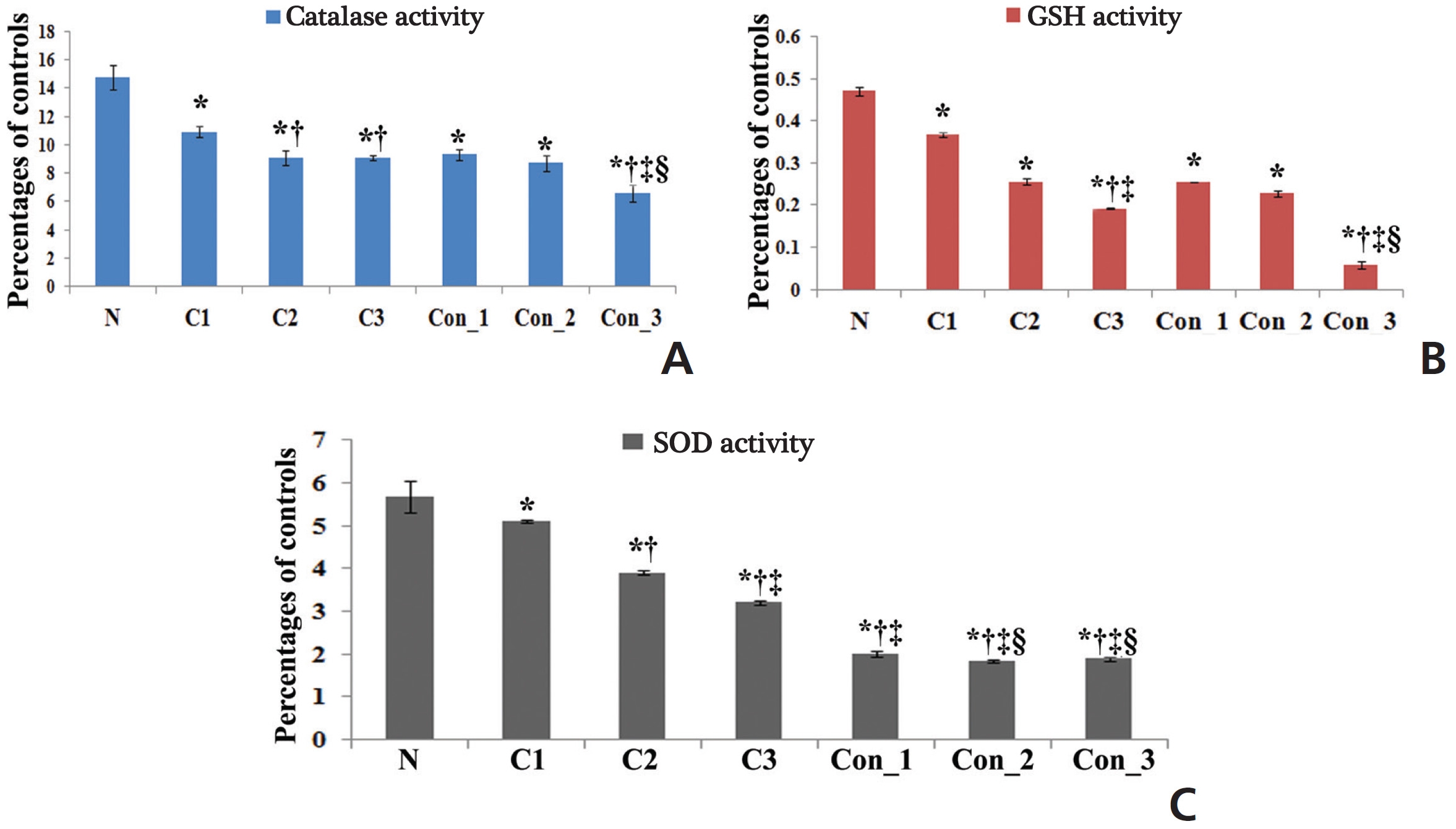
The accumulation of ROS was found to be slightly higher in the perfused lung epithelial cells of lung-cancer-bearing groups than in those of the normal group, which gradually increased with increasing post-cancer interval, presumably due to an increase in stress after BaP induction. However, the ROS generation was surprisingly higher in Condurango-treated lung epithelial cells, especially during the 7th month (Fig 2). This result would suggest that Condurango has the capacity to generate ROS, which may act to reduce the antioxidative activity and induce oxidative stress-mediated cancer cell death. The antioxidant activities of glutathione (GSH), catalase and superoxide dismutase (SOD) were found to be slightly lower in -cancer- bearing rats than in normal rats (Fig 3). However, in Condurango-treated groups, antioxidative activities were significantly lower, especially at the 7th month, which further suggests the ROS-generation capacity of Condurango by inhibiting antioxidants.
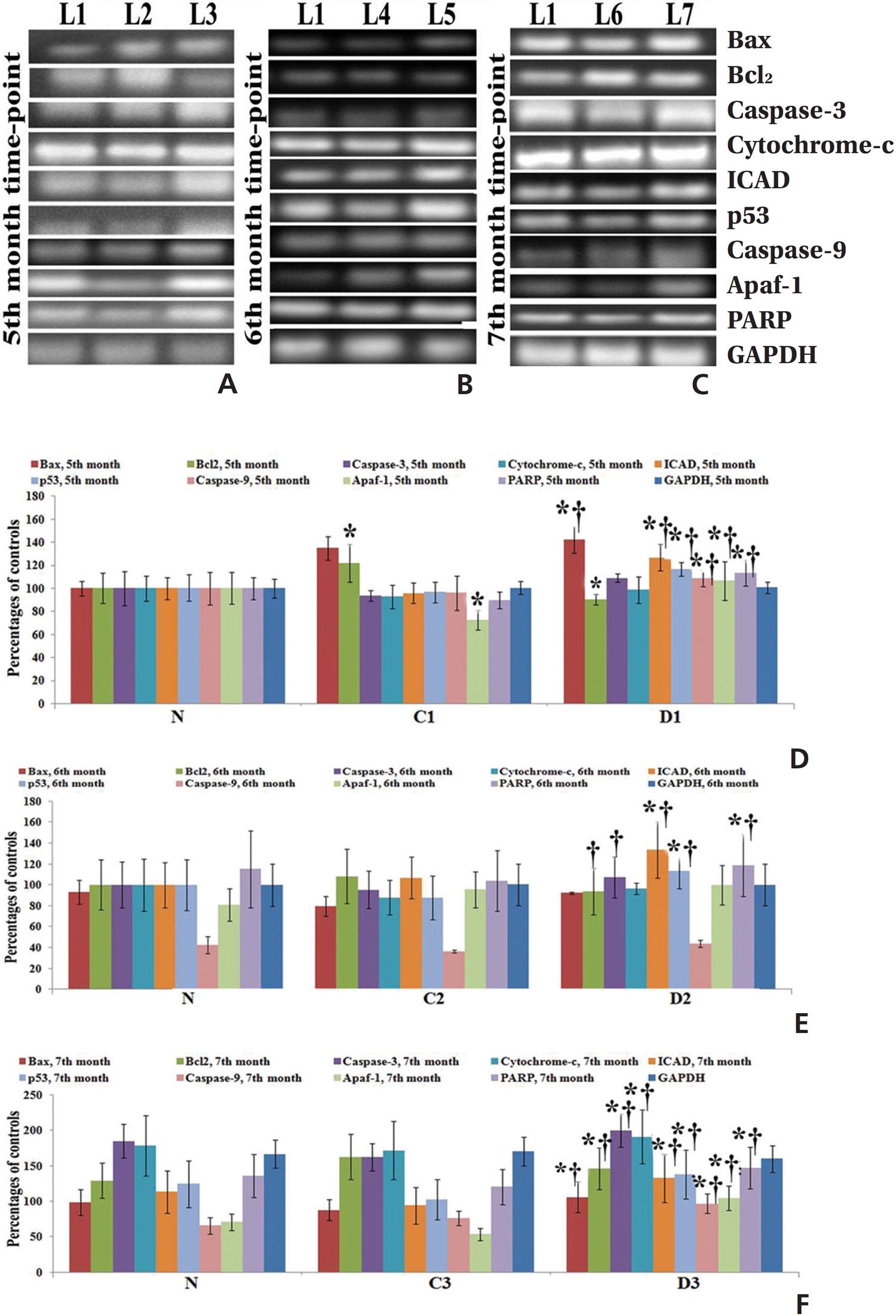
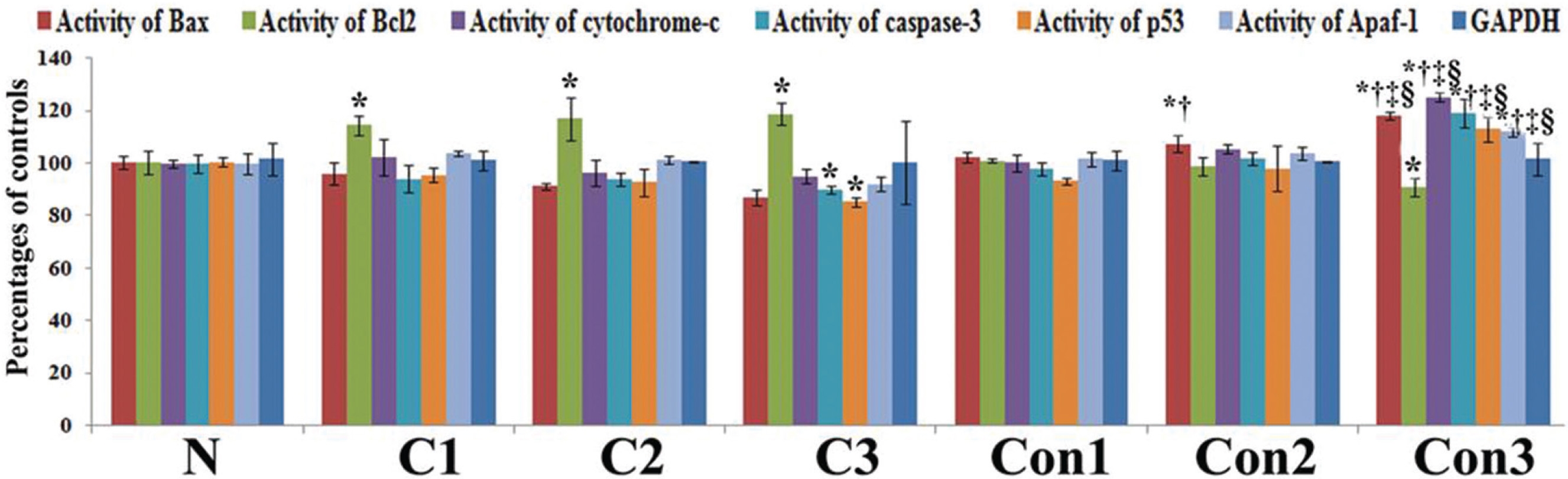
mRNA expressions of different apoptotic markers showed significant levels of changes (up regulation of pro-apoptotic genes and down regulation of anti-apoptotic gene) at the 7th month in the Condurango-treated group with respect to the normal and the cancer-bearing groups (Fig 4).
Protein expressions of different apoptotic markers were quite significant after three months of Condurango treatment with respect to the normal and the cancer-bearing rats, a fact which was also supported by the study of the mRNA level (Fig 5).
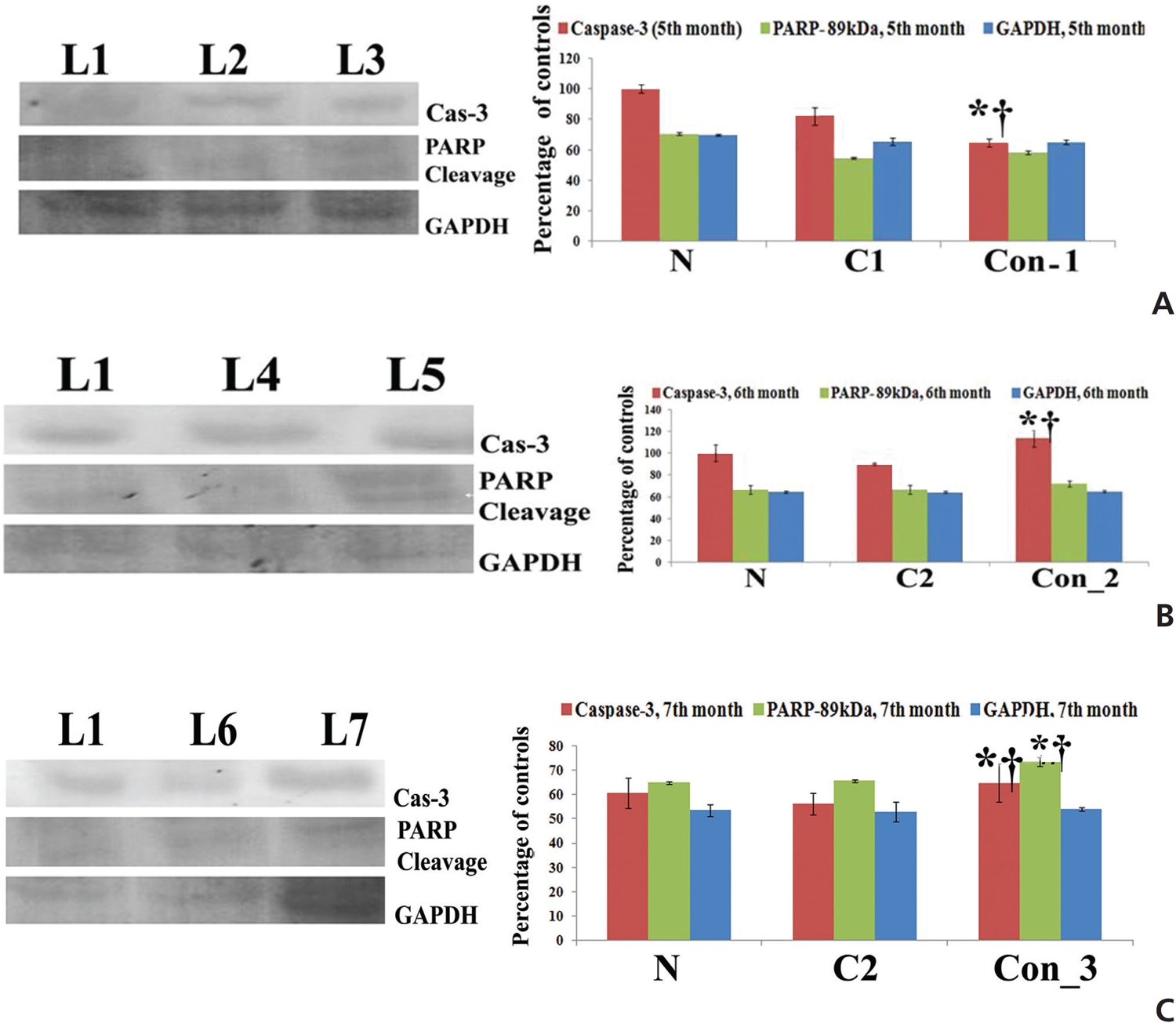
The results showed up regulation of caspase-3, especially at the 7th month time point in the Condurango-treated sample as compared to those of the normal and the cancer controls; this would suggest apoptosis was mediated mainly through a caspase-3-mediated pathway in the lung-cancer-bearing rats (Fig 6).
PARP activation is marked by cleavage at the 89 kDa (active) and the 116 kDa (inactive) polypeptide subunits. Results showed that the expression of the active fragment (89 kDa) was up regulated in Condurango-treated samples, especially at the 7th month time point (Fig 6). This study further provides a good indication of apoptosis initiation via caspase-3 activation and PARP cleavage, which can promote DNA fragmentation at the end of apoptotis.
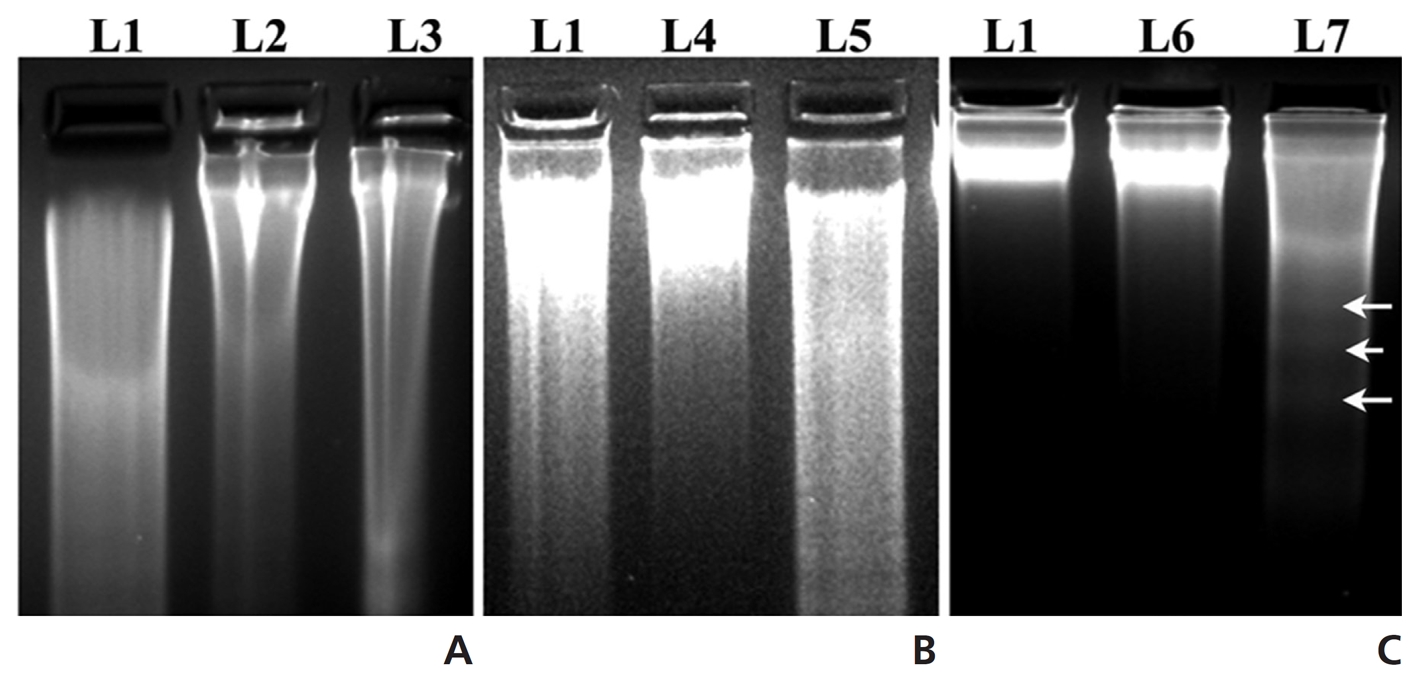
(Fig 7) indicates the formation of inter-nucleosomal DNA fragmentation, which was very much prominent in the DNA of the Condurango-treated group for three months with respect to those in the normal and the drug-untreated groups.
Our preliminary study on Condurango in H522 and A549 cells gave a positive hint towards the apoptosis-inducing potential of Condurango via DNA damage [20]. For further confirmation, an enquiry was conducted using BaP-induced lung cancer in rats. In cancer-bearing rats,alveolar spaces were gradually condensed and became stiff, presumably due to cancer cachexia. However, after post-cancer Condurango treatment for different periods, considerable recovery was noticed, particularly at the 7th month time point. This result indicates an anti-neoplastic property of Condurango, which requires a rather longterm treatment.
One of the major reasons behind BaP-induced lung cancer is a reduction in the antioxidant defense mechanism, which is related to a reduction in the activities of anti-oxidative enzymes including SOD, catalase and GSH [24]. These antioxidants can synergistically scavenge ROS. SOD can disrupt superoxide radicals and protect the cells against superoxide. Several reports have recently cited decreased activities of SOD and catalase under various carcinogenic conditions. Catalase is widely distributed in all tissues and is known to catalyze the breakdown of hydrogen peroxide produced by tumor cells. Changes in the rate of cancer cell proliferation are accompanied by changes in their intracellular GSH levels; consequently, these could be reflected in their antioxidant machineries. SOD, catalase and GSH constitute a defense group against ROS, and their levels were decreased in BaP-induced lung-cancer-bearing animals [25]. Decreases in the activities of antioxidants were observed in cancerous rats, but in Condurango-treated rats, antioxidative activities were significantly lower, especially at the 7th month, which would suggest the antioxidant inhibitory potential of Condurango. Accumulation of ROS was found to be slightly higher in lung-cancer-bearing groups than in normal ones due to induction of stress generated by BaP-intoxication, but ROS was surprisingly higher in Condurango-treated lung epithelial cells, especially at the 7th month. This result would suggest that Condurango has a ROS induction capacity, which may act to reduce antioxidant activities and induce oxidative-damage-mediated cancer-cell death.
Apoptosis-mediated cell death through target-specific activity has destructive effects on cancer cells. Apoptosis is mainly regulated either via an extrinsic pathway or via a mitochondria-dependent intrinsic pathway. Apoptotic regulation mainly depends upon various gene products, including pro-apoptotic (p53, Bcl-2, and Bax) and anti-apoptotic genes (Bcl-2, Bcl-XL). On the other hand, over-expression of Bax results in an enhancement of ROS and facilitation of cytochrome-c release via mitochondrial membrane depolarization [26]. In this regard, mitochondria play a key role through cytochrome-c release and caspase-3 activation. In this mitochondria-dependent pathway, the Bcl-2 family members govern the release of cytochrome-c and the activations of apaf-1, caspase-9, 3 and PARP that initiate DNA fragmentation and apoptosis.
Up regulation of Bax, cytochrome-c, p53, caspase-3, caspase-9, apaf-1, ICAD, PARP and down regulation of the anti-apoptotic gene Bcl-2 at the mRNA level after three months, especially at the 7th month time point, of post-cancer Condurango treatment against normal and cancerous groups suggest an anti-cancer potential of Condurango through apoptosis induction for long-term post-cancer treatment. The analysis of expressions of the apoptotic markers at the protein level (through ELISA) also supported the related mRNA expressions. As caspase-3 is the major apoptotic marker involved in the intrinsic pathway, the expression of caspase-3 was further analyzed by using a western blot. Results clearly depicted significant up regulation of caspase-3, specifically at the last month of post-cancer Condurango treatment, supporting our in-vitro findings [12]. The caspase-3-mediated intrinsic apoptotic pathway was completed by formation of PARP cleavage at the 89 kDa (active) and the 116 kDa (inactive) subunits and simultaneous occurrence of DNA fragmentation [27]. The western blot analysis revealed the formation of PARP cleavage with a significant increase at the 89 kDa subunit and simultaneous occurrence of DNA fragmentation in Condurango-treated groups, especially at the 7th month. These results finally suggest the apoptosis-inducing potential of Condurango for long-term post-cancer treatment, in vivo, via a ROS-dependent caspase-3-mediated intrinsic pathway.
The results of this study are encouraging enough to undertake further animal experimentation with higher mammals so that the effects of Condurango as a potent anti- lung cancer agent and its safe use by CAM practitioners in human patients can be verified.