Cancer is a hyperproliferative disorder that involves morphological cellular transformation, dysregulation of apoptosis, uncontrolled cellular proliferation, invasion, angiogenesis, and metastasis [1]. As the disease runs its course, the human body, which is made up of several components that together make a system, is in a constant cycle of attack, battle, and recovery. Numerous studies have recognized an association between the key immune, coagulation and inflammatory factors and the biological status of the body during the processes [1-3]. NK cells, which are innate immune system cells, are localized in peripheral blood, lymph nodes, the spleen, and bone marrow. NK cells constitutively express a lytic mechanism able to kill target cells independently from any previous activation. While the cytotoxic T-cells recognize infected cells by their peptides, the major histocompatibility complex (MHC) class I molecules present, NK cells look for the absence of MHC class I molecules. One proposal is that NK cells complement cytotoxic T-cells by taking charge in situations where MHC class I expression is hindered through the effects of virus infection [4]. Once NK cells are activated, they release small cytoplasmic granules of proteins called perforin and granzyme, which cause the target cell to go through apoptosis. These functional features have suggested a role for NK cells in the control of tumor growth and metastasis.
Fibrinogen, the major component of clots also has important roles in angiogenesis. Tumor-associated blood vessels are typically leaky; hence, fibrinogen and other clotting molecules extravasate from the defective vessels and form fibrin deposits around the tumor. These deposits provide a pro-angiogenic matrix that facilitates endothelial cell adhesion, migration, proliferation, and differentiation into tubules [5]. Formation of the fibrin matrix allows the malignant cells to adhere to and invade the tissues in metastatic sites and protects the cancer cells from immune surveillance [6-8]. The fibrin matrix also supports the migration of tumor cells and provides a scaffold for the formation of new blood vessels [9].
Hematology lab testing follows the numbers of cells in blood, including red blood cells (RBC), white blood cells (WBC), platelets and others. Symptoms such as anemia, infection, and abnormal bleeding are looked for to determine how the body is dealing with the chemotherapy drugs or radiation treatments and to follow up on the general state of the patient’s health. Studies report that Korean Medicine (KM) treatments have anti-coagulative effects under hyper-coagulative conditions [10-12], and correlations between KM treatment and the promotion of immune response have also been reported [13-15]. With the cross-link between inflammation, immunity, coagulation, and angiogenesis playing important roles in cancer progression and the treatment prognosis [1-3], the effect of KM inpatient care at the East-West Cancer Center (EWCC), Dunsan Korean Medical Hospital of Daejeon University, was investigated by following the cancer patients’ hematological lab data and their quality-of-life reports.
The study population consisted of inpatients who were admitted to the East-West Cancer Center, Dunsan Korean Medical Hospital of Daejeon University, Korea, from March 1, 2011 to August 31, 2011. Patients were histologically or cytologically diagnosed with various cancers and received wheel balanced cancer therapy (WBCT), the center’s inpatient multi-modality Complementary and Alternative Medicine (CAM) cancer program, for durations ranging from 9 to 34 days. A total of 80 patients, 57 females and 23 males with a mean age of 48.95 years (range: 27 – 82 years), were followed, and routine laboratory screenings were performed. All variables, including demographic, clinical and pathological findings, were recorded. The mean hospitalization period was 16.75 days. As a retrospective follow up, all data were collected as part of routine clinical practice. All treatments were conducted with prior patient consent and permission was obtained to use or disclose their health information for future research purposes.
All patients received EWCC’s multi-modality CAM cancer care program, WBCT. WBCT incorporates various treatment modalities under four sub-programs: (1) anti-cancer nutrition; (2) metabolism activation: acupuncture, pharmacopuncture, thermotherapy, massage, physical therapy, and hydrotherapy; (3) herbal therapy: EWCC’s signature anti-angiogenic and immune enhancing herbal prescriptions, which include Hang-am plus (ingredients:
Emphasis was placed on cancer-coagulation factors, immunity, and patients’ functional status. The lab reports, including NK cell count (CD16 + CD56), fibrinogen, WBC, lymphocytes, monocytes, neutrophils, RBC, hemoglobin, platelets, ESR, and Eastern Cooperative Oncology Group (ECOG) performance status values [16], which were recorded before and after hospitalization, were closely monitored.
Patients were divided into 3 groups depending on the status of their treatment: prevention of recurrence and metastasis group (prevention group), Korean medicine treatment only group (OM Tx only group), and combination of conventional and Korean medicine treatment group (combination group). The prevention group included cancer-free patients who were under surveillance for further disease progression. The KM Tx only group and the combination group included patients with cancer who were undergoing either Korean medicine treatment or combined treatment with both Korean medicine and conventional medicine.
Results are given as mean values ± standard deviations (SDs). For continuous variables, all statistical analyses were performed using SPSS version 12.0 for Windows (SPSS, Chicago, Ill, USA). The paired
3.1. Laboratory changes in patients
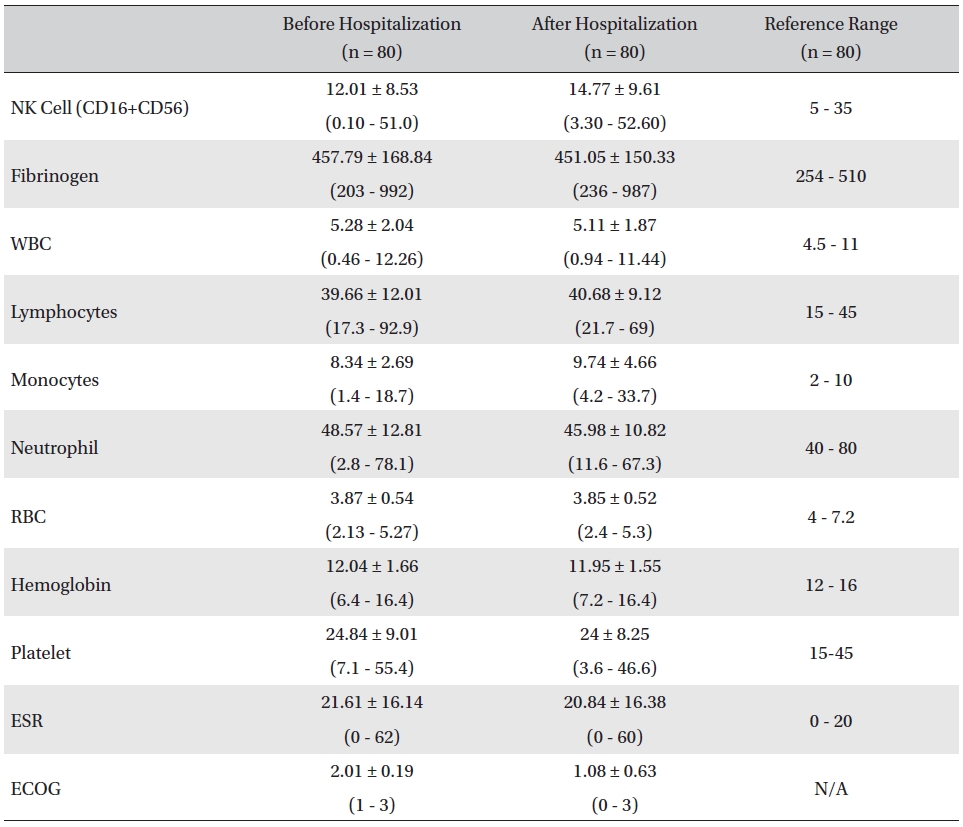
The mean lab values checked before and after hospitalization of all cancer patients who received inpatient care at the EWCC showed minimal changes in most measures. The ranges of the lab values showed slight tendencies to move closer to the reference ranges, but showed no remarkable changes. The mean ECOG performance status of the patients dropped from 2.01 ± 0.19 before to 1.08 ± 0.63 after hospitalization. The bottom status for patients after hospitalization was 0, fully active and able to carry on all pre-disease activities without restriction, whereas the bottom status for patients before hospitalization was 1, restricted in physically-strenuous activity but ambulatory and able to carry out work of a light or sedentary nature (Table 1).
[Table. 1] Changes in lab values and ECOG before and after inpatient care
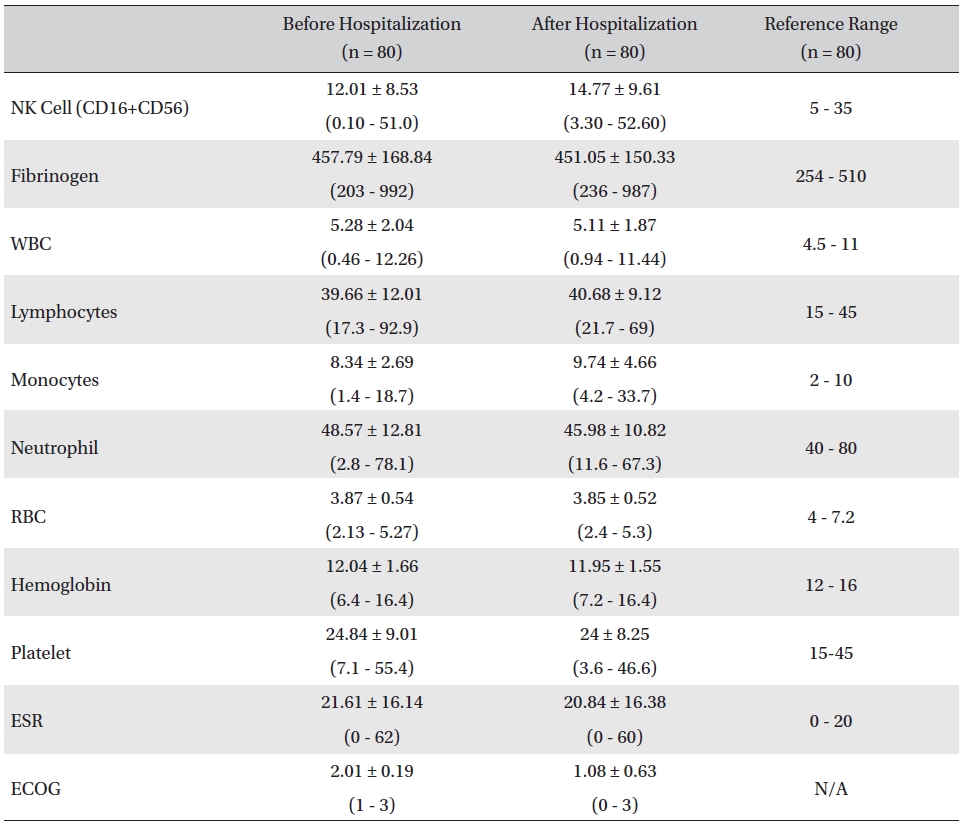
Changes in lab values and ECOG before and after inpatient care
3.2. Comparison of changes in assessment groups
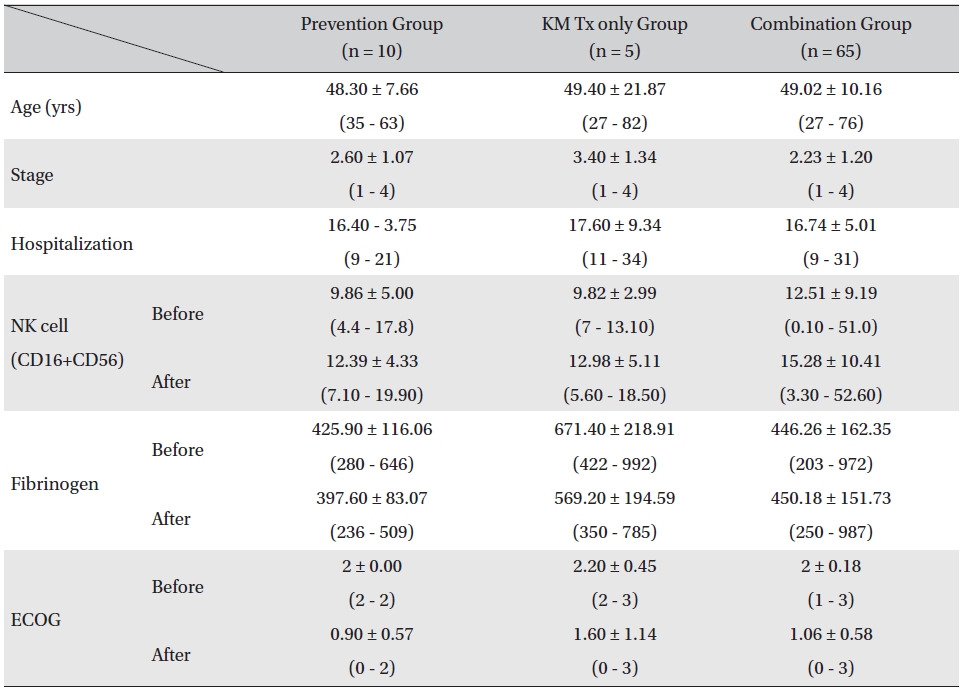
Patients were divided in to three assessment groups depending on their treatment phases, prevention group, KM Tx only group, and combination group, and changes in the NK cell, fibrinogen count, and ECOG performance status values were investigated. The mean ages of the patients for all 3 groups were similar (prevention group: 48.30 ± 7.66 years, KM Tx group: 49.40 ± 21.87 years, combination group: 49.02 ± 10.16 years) as were the mean hospitalization days (prevention group: 16.40 ± 3.75 days, KM Tx group: 17.60 ± 9.34 days, combination group: 16.74 ± 5.01 days). The mean stages of the patients differed by groups, with a higher mean stage being found in the KM Tx only group (3.40 ± 1.34) than in either the prevention group (2.60 ± 1.07) or the combination group (2.23 ± 1.20) (Table 2).
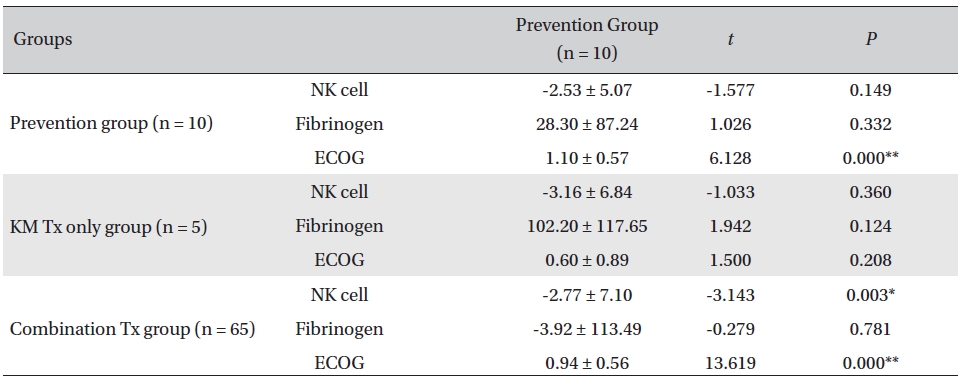
Overall, NK cell count increased in all groups. The highest mean increase was seen in the KM Tx only group (from 9.82 ± 2.99 to 12.98 ± 5.11, with -3.16 ± 6.84 paired difference). However, the difference was not measurably greater than it was in the other two groups. The NK cell count change in the combination Tx group was statistically significant (
[Table. 2] Comparison of patient characteristics, lab values, and ECOG between assessment groups
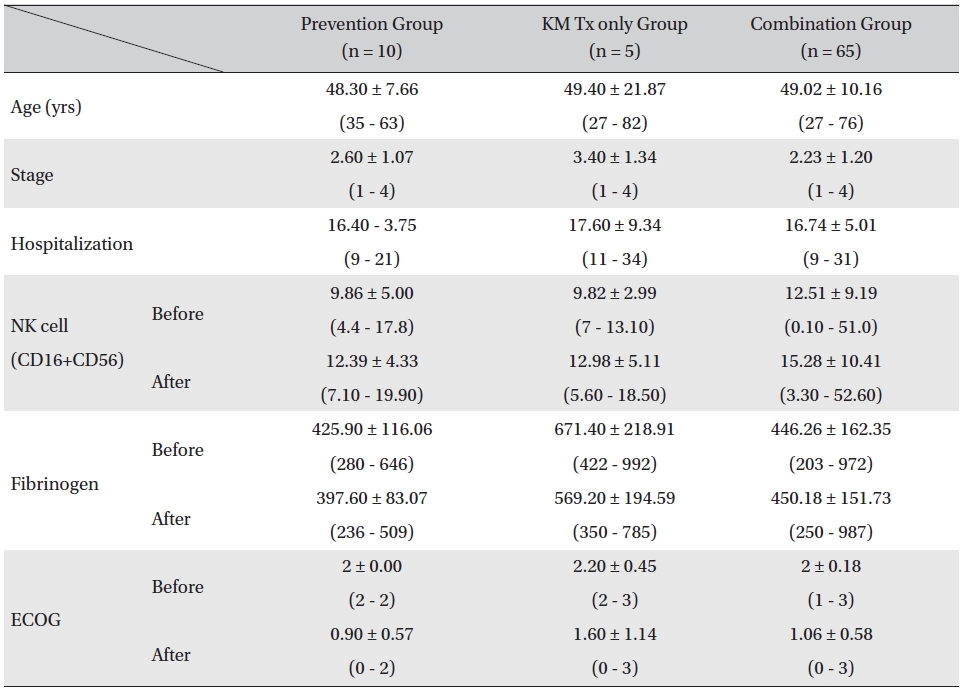
Comparison of patient characteristics, lab values, and ECOG between assessment groups
[Table. 3] Comparison of NK cell count, fibrinogen, and ECOG changes between groups
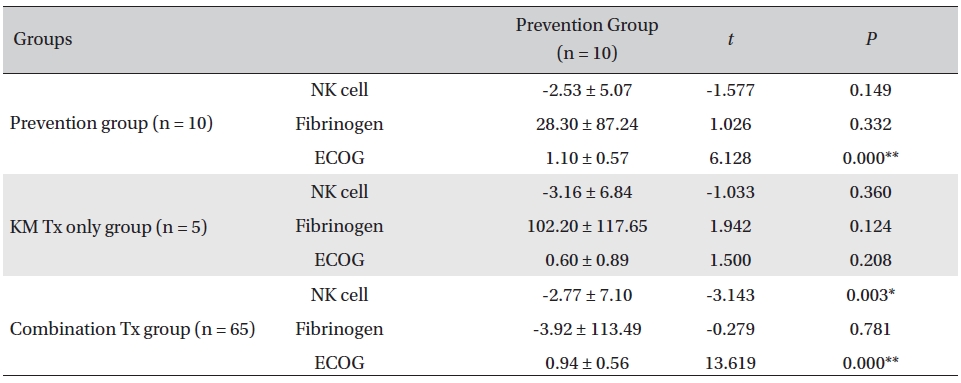
Comparison of NK cell count, fibrinogen, and ECOG changes between groups
The relationship between NK cell count and cancer progression is controversial, and the results of research have been conflicting [17-19], even though more research tends to show a positive correlation between low NK cell count and cancer. Plonquet et al. found the peripheral blood natural killer cell count to be associated with clinical outcome in patients with aaIPI 2–3 diffuse large B-cell lymphomas [20]. Stewart et al. found the CD3-16+56+ cell count to be an independent predictive factor for autologous blood stem cell mobilization (BSCM) in the heterogeneous group of cancer patients [21]. Studies on NK cell activities in mediation of cancer immunity also support the correlation between the NK cell count and cancer progression [22-24].
Recent evidence from several laboratories has linked malignant transformation, tumor angiogenesis, and metastasis to thrombus formation, mediated perhaps by signaling cascades that can be triggered in a clotting-dependent and/or clotting-independent manner [25-27]. The precise role of the cytoplasmic domain of tissue factor (TF), which has been targeted as the likely signaling region of the molecule, remains controversial [28, 29]. TF is a 47-kDa transmembrane protein that initiates the coagulation cascade. Similar functions have been suggested for TF and vascular endothelial growth factor (VEGF). TF and VEGF have been found to be co-localized on malignant cells from human lung cancer and breast cancer samples [30]. The increased expression of TF in tumors results in an angiogenic phenotype by up regulation of the pro-angiogenic factor VEGF and down regulation of the anti-angiogenic factor thrombospondin [31]. An aberrant increased expression of TF is known to occur in a variety of tumor types, including breast cancer, leukemia, glioma, non-small-cell lung cancer, and colon cancer [32-36]. Increased levels of TF have been associated with increased angiogenesis, advances stages of disease, and poorer outcome [36].
In our study, a decrease of ECOG was seen in all groups, implying general improvement of performance status in all the patients. The largest decrease was seen in the prevention group, and the smallest decrease was seen in the KM Tx group. This result was presumably related to the different concentrations of late-stage-cancer patients between the groups. The data from the present study revealed a general decrease in the fibrinogen count, fluctuating NK cell counts, and enhanced ECOG performance status in all groups. The NK cell count of CD16+56 increased in all assessment groups after the CAM inpatient care, though not by much. The KM Tx only group showed the largest increase, but the differences between groups were not enough to say that the treatment received had had any effect on the overall NK cell count. On a closer look, the NK cell count fluctuated with treatment in individual patients and did not seem to show any consistent correlation with the treatment or the functional improvement; this was presumably due to tumor escape by way of alteration of NK cell function and to the resistance to killing associated with tumor progression and chronic inflammation. The level of the clotting factor fibrinogen decreased most in the KM Tx only group, despite that group’s having higher mean cancer stages and worse progression. That increased by a minuscule amount in the combination group, which is thought to have been caused by the multiple mechanisms of concurrent cytotoxic conventional therapies causing clotting response and of Korean medicine treatment fighting off the coagulation at the same time.
Despite the short follow-up time, the results support the positive role of the multi-modality KM-based inpatient care program in complementing the disease treatment course by lowering the cancer coagulation factor fibrinogen and improving the patients functional status. However, its correlation with changes in the NK cell count is not clear. In the future, further studies that have longer treatment durations and use more diverse follow up of the cancer immune, coagulation and inflammation factors as measures to accurately evaluate the efficacy of the treatment are needed.