Pharmacopuncture refers to be in the limelight acupuncture therapy that treat diseases by using techniques such as warming (Chunghwa), alcohol extraction or pressing to extract drugs from one or more oriental medicines and then injecting them into specific acupoints related to the disease and adjusting meridian functions [1].
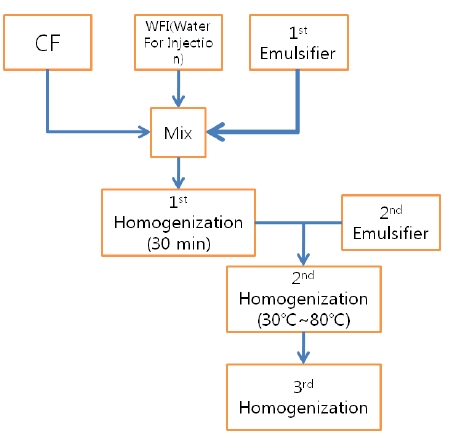
CF was prepared in a sterile laborotary at the Korean Pharmacopuncture Institute (KPI). After the seeds had been cleaned, ground and peeled, they were screw pressed on a prepared mat. Rough unwanted residue was removed, and natural precipitation was induced. The extract from a 3-step filter was packaged with nitrogen [3]. Water soluble Carthmi-Flos herbal acupuncture (WCF) was prepared by homogenizing CF, water for injection (WFI) and an emulsifier.
Pharmacopuncture with
Lim
The WCF was provided by the KPI. WCF was developed to increase the treatment effects by increasing absorptivity and to reduce the side effects by reducing residue of existing CF. WCF was prepared in three steps: In step 1, CF, WFI and primary emulsifier were mixed together and homogenized for 30 minutes by using a high-speed homogenizer. Emulsifier was added in step 2 and homogenized at 30-80℃. In step 3, the temperature was reduced, and the mixture was homogenized to yield WCF. (Fig. 1).
Sprague-Dawley rats, purchased from Orientbio Inc., Korea, were used as experimental animals. The rats, 24 males and 24 females, were 5-weeks old with body weights of 111.8-135.8 g and 107.4-127.9 g, respectively. The animals were given a 7-day grace period to acclimatize before any measurements. General symptoms and body weights were observed to verify that no problems existed with the animals. During the grace period, individual subjects were marked, and at the end of the grace period, 20 males and 20 females closest to the standard body weight were selected and randomly divided into 4 groups of 5 male rats and 5 female rats so that the mean weights of the four groups were uniform.
Solid feed (Teklad Certified Irradiated Global 18% Protein Rodent Diet 2918C) and filtered tap water (Cheongju tap water filtered using a filter water sterilizer and irradiated with ultraviolet rays) were freely available. The breeding environment was as follows: a temperature of 20.0℃- 22.8℃, relative humidity of 48.5%-65.8%, 10- to 15-hour ventilation, 12-hour/day lighting cycle, and illuminesence of 150-300 Lux.
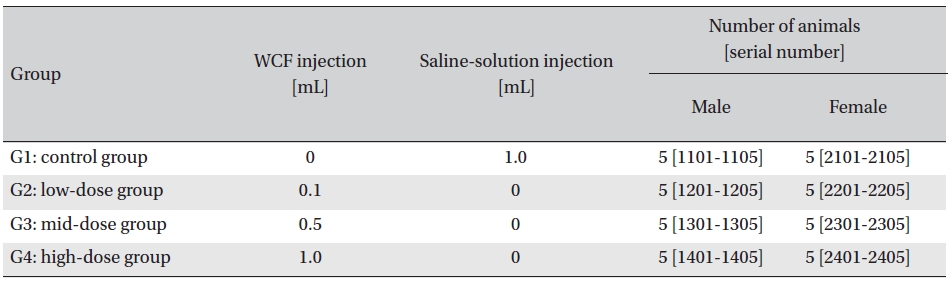
Intramuscular dose was selected because WCF is expected to be clinically applied to muscles. The clinical dose of WCF was 1.0 mL per application, as no fatalities had been observed at doses up to this value in preliminary tests (Biotoxtech Study No.: B12873P) on male and female rats. Thus, under consultation with the test’s requester, 1.0-mL/ animal was configured as the high dose. Medium and low doses were 0.5- and 0.1-mL/animal; the same amount of saline solution, 1.0-mL/animal, was injected into the control group. (Table 1)
[Table. 1] Groupings of the rats
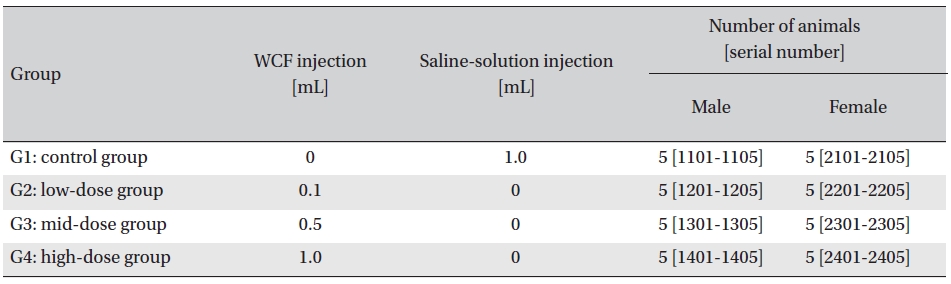
Groupings of the rats
Injection was performed using a disposable syringe (1 mL, 26 G). Single doses were injected into the left femoral muscle for the low-dose and the medium-dose groups. For the control and the high-dose groups, single injections, 0.5 mL/site, were done into the left and the right femoral muscles. On the day of injection (day 0), clinical symptoms and fatalities were observed at 30 minutes and at 1, 2, 4 and 6 hours after injection. From day 1 to day 14, general symptoms were observed once a day. Body weights were measured (CP3202S scale) on days of injection (before injection) and on days 3, 7 and 14. For the hematology test, blood was collected from the abdominal aorta on day 15 after applying anesthesia by isoflurane. Hematological analyses were carried out by placing 1 mL of collected blood into a tube containing ethylenediaminetetraacetic acid, which was then analyzed using a blood corpuscle analyzer (ADVIA 120, SIEMENS, Germany). For the coagulation test, about 2 mL of collected blood was placed in a tube containing 3.2% sodium citrate and centrifuged for 10 minutes at 3,000 rpm, after which the blood plasma was collected. Measurement was done using a coagulation time analyzer (Coapresta 2000, SEKISUI, Japan). The prothrombin time and the activated partial thromboplastin time (APTT) were measured. After completing the hematology tests, for the clinical chemistry test, we centrifuged the remaining blood for 10 minutes at 3,000 rpm and collected the blood serum. A clinical chemistry analyzer (7180, Hitachi, Japan) and an electrolyte analyzer (AVL9181, Roche, Germany) were used.
Visual inspection of all body organs and tissues was performed on all animals after necropsy. Body organs and tissues were extracted and fixed in 10% neutral buffered formalin, and histopathological observations were carried out.
Body-weight, hematology and clinical chemistry results were tested using SAS (version 9.3, SAS Institute Inc., USA). Bartlett tests were performed for homo-scedasticity (significance level: 0.05). For homo-scedasticity, one-way analysis of variance tests were performed to yield the significance (significance level: 0.05), and multiple Dunnett’s
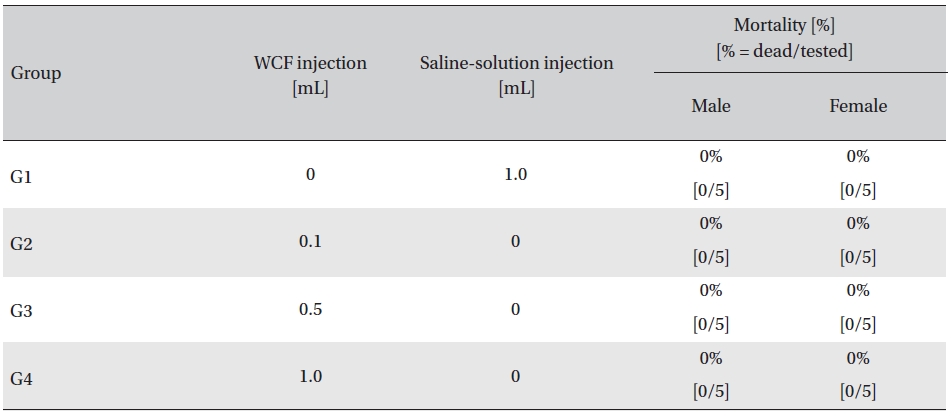
In this study, no mortalities were caused by injecting intramuscular doses of WCF. Accordingly, computation of the LD50 was impossible; however, a single intramuscular dose of WCF at 1.0 mL/animal caused no mortalities. (Table 2)
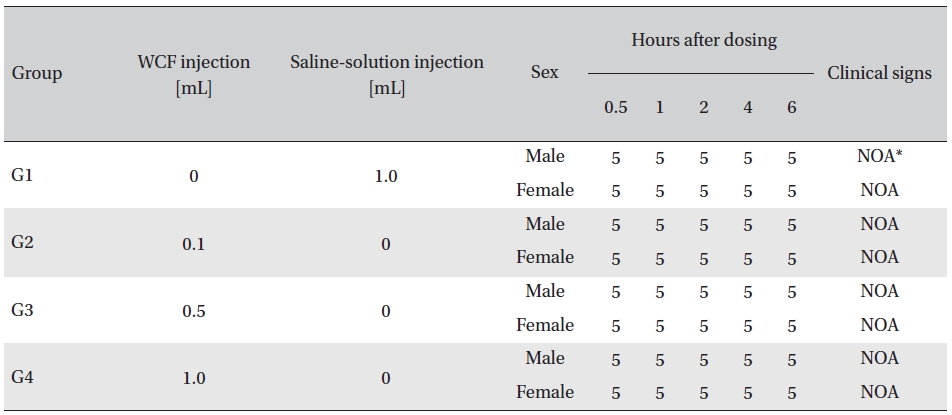
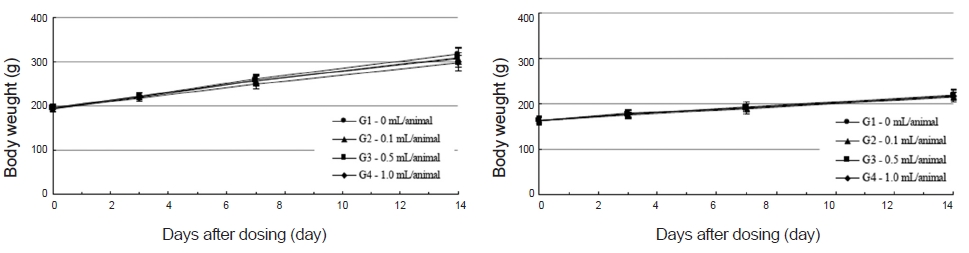
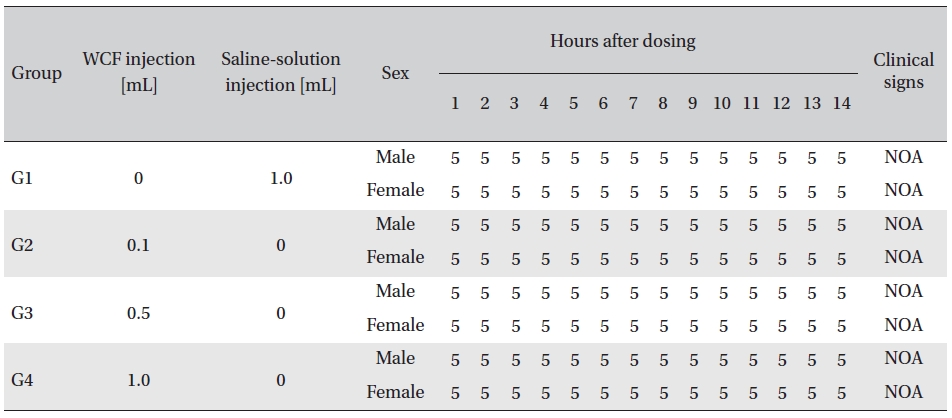
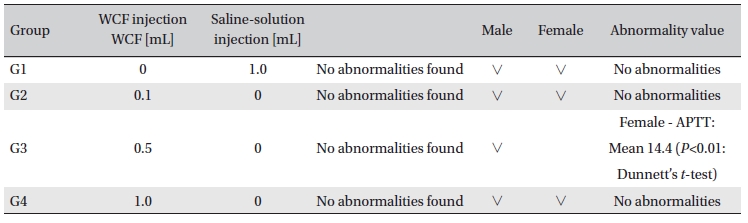
No abnormal clinical symptoms (type of toxicity symptom, time of expression, time of recovery, etc.) were observed on the day of injection (day 0) at 30 minutes and at 1, 2, 4 and 6 hours after injection, as was the case for days 1 to 14 after injection. All groups showed a continued increase in body weight, but the differences among the groups were not significant. (Fig. 2. Tables 3,4,5)
Hematological analyses on the rats’blood showed WCF to have no effect. However, APTT (sec) mean of the G3 female group showed a statistically significant change (
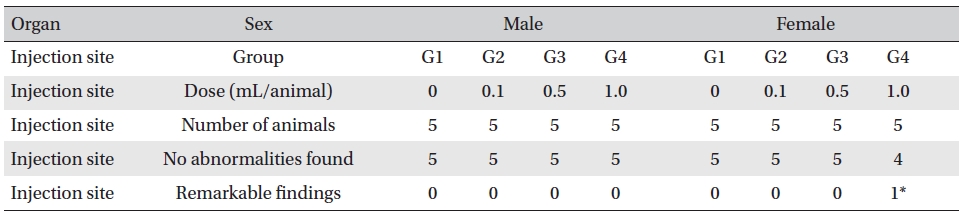
On the histopathological tests, one female in the highdose group showed infiltration of mononuclear cells and a multi-nucleated giant cell around eosinophilic materials caused by WCF in an area similar to a cross section of muscular fiber, and 8 eosinophilic materials were clustered at a local part. No abnormalities were observed in any other subjects. (Table 9)
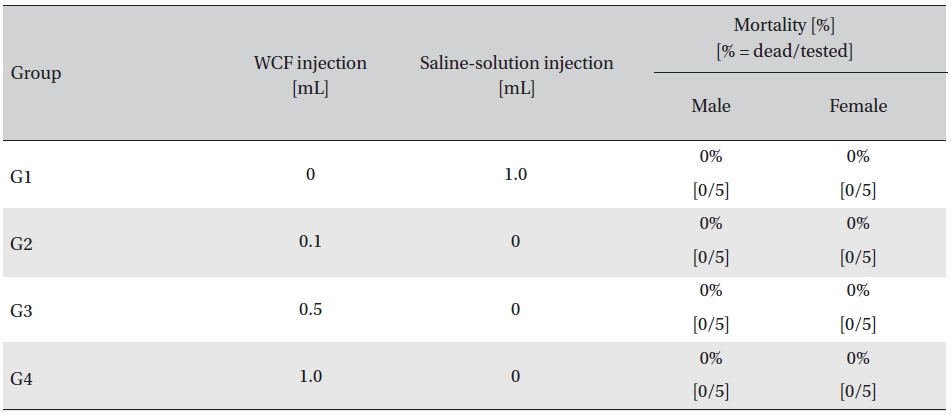
Mortality
[Table. 3] Clinical signs on day 0
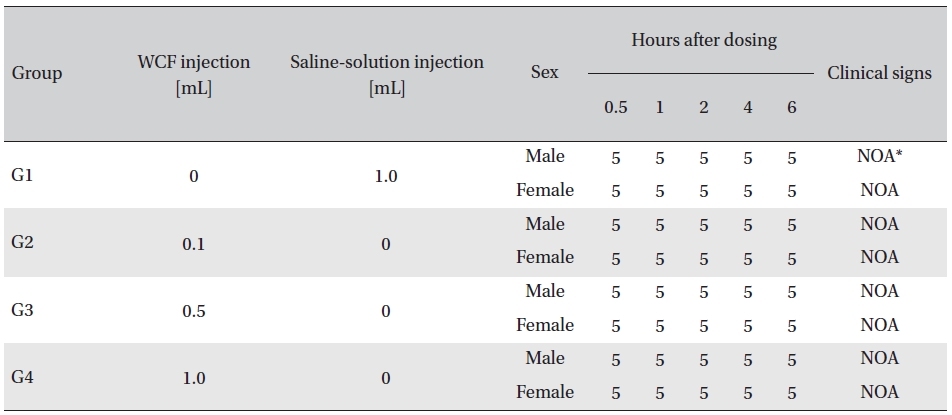
Clinical signs on day 0
WCF is prepared by homogenizing a mixture of CF, WFI and emulsifier and increases the treatment efficacy while maintaining the characteristics of existing CF. The effects of CF have been demonstrated by various studies; i.e., CF can be used for pain relief, is effective for a variety of different pains [13], increases regional cerebral blood flow significantly [14], and can be used in a variety of different fields with different effects such as anti-carcinogenic [7, 12] and anti-oxidative [15] effects.
In this experiment, an intramuscular-dose toxicity test of WCF was performed by Biotoxtech to evaluate the safety of WCF manufactured by the KPI according to the GLP. No mortalities occurred in any group. Also, observations taken after injection of WCF showed no abnormalities in the clinical symptoms of the four groups. No significant bodyweight differences were observed in any of the groups. Hematologically, a change in the APTT (sec) mean value of the G3 female group was observed, but had no clinical or toxicological meaning. Clinical chemistry tests showed no differences between the control and experimental groups. No visual abnormalities were observed in any group at necropsy. Finally, histopathological observation showed that WCF caused a change in one female in the high-dose group. Visual examination showed the subject to have suffered infiltration of mononuclear cell and to have had a multi-nucleated giant cell around eosinophilic material. No abnormalities were observed in any other subjects.
Studies on the side effects and the safety of CF are continuously being conducted. According to [3], pain, flare and edema were found in the surgical parts in patients after CF therapy. After 4 days, side effects, such as flare and edema, mostly disappeared. They were thought to have occurred due to a mixture of highly-toxic drugs in the oil press during CF during preparation [3]. Side effects created by CF were reported in [16]. Suppuration and pain appeared in the surgical parts in 1 patient who received CF therapy of 0.1 mL/1 time/week for 8 months and 1 patient who received CF therapy of 0.1 mL/1-3 times/week for 3 months.
[Table. 4] Clinical signs from day 1 to day 14
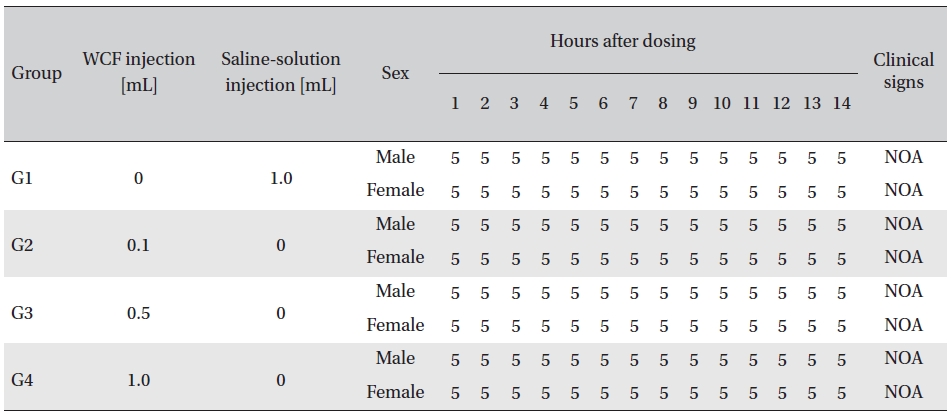
Clinical signs from day 1 to day 14
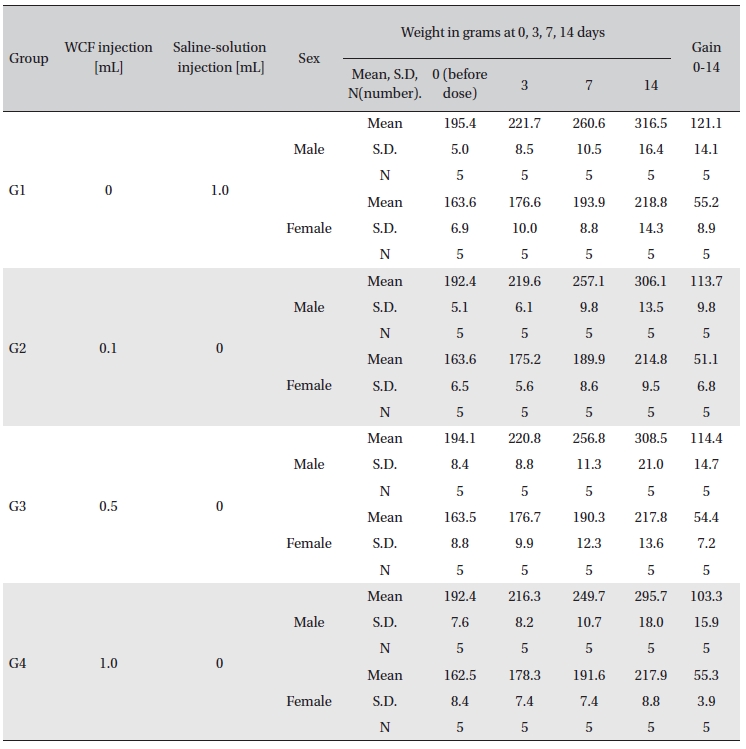
Body weights
[Table. 6] Hematology findings
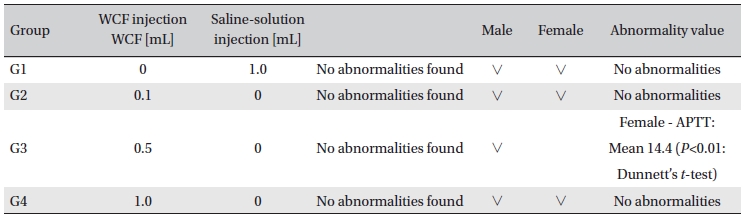
Hematology findings
[Table. 7] Clinical chemistry findings
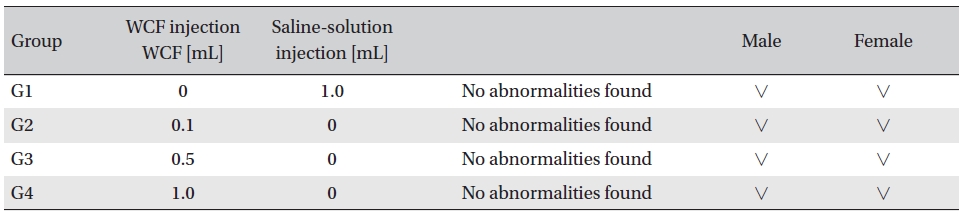
Clinical chemistry findings
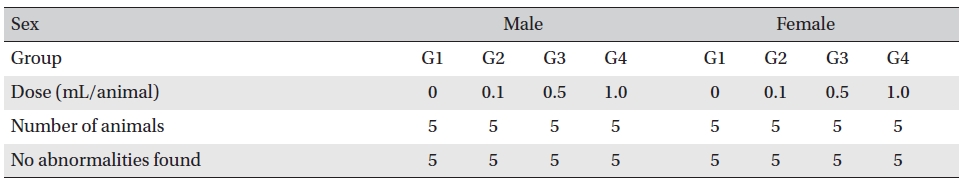
Necropsy findings
[Table. 9] Histopathological change findings
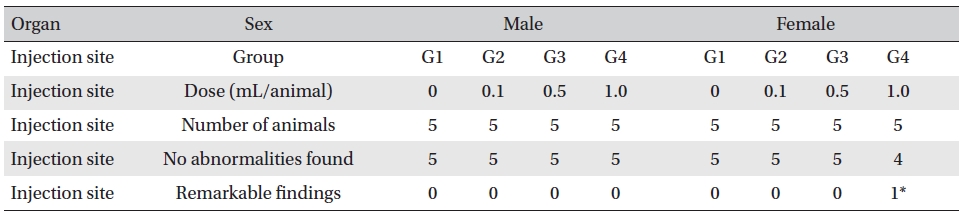
Histopathological change findings
Unlike in [3], the side effects were deemed to be due to an accumulation of CF in the human body from long-term therapy [16]. The safety of CF was reported in [12] based on a toxicity test of CF, but this was only a short-term study on acute and subacute toxicity. Chronic toxicity tests on CF must be carried out in the future.
Although histological abnormalities caused by WCF were found in a subject of the high-dose group in this experiment, according to [17], no histological abnormalities were observed in Sprague-Dawley rats treated with CF 3 times a week for 1-2 weeks. However, the group with 4 weeks of therapy showed histological ambiguity regarding the boundary of muscular tissues, observation of flares, and induction of inflammations [17]. A histological study with a greater number of subjects and a longer period is deemed necessary to examine long-term or high-dose effects of CF or WCF.
The safety of CF was demonstrated, but its side effects were presented in several studies. However, studies on the safety and the side effects of WCF are lacking. Thus, the toxicity and the safety of WCF must be investigated, and long-term histological studies based on large numbers of subjects are deemed necessary.
The results of single intramuscular-dose toxicity tests with WCF on 4 groups of Sprague-Dawley rats, 3 experimental groups (0.1 mL/animal, 0.5 mL/animal and 1.0 mL/animal) and 1 control group (1.0 mL/animal of saline solution), are as follows:
This study do not show WCF’s safety of human body. To examine the safety of WCF, we need to carry out testing to check whether or not the toxicity would have negative effects on human body.