Marine benthic dinoflagellates are of tremendous global economic significance. At present, benthic dinoflagellates are receiving much attention in terms of microalgal monitoring due to the problems encountered with potentially harmful benthic or bentho-planktonic species which lead to the economic loss by killing fish/shellfish through toxic blooms and subsequently human health in subtropical to tropical coastal areas. The most well known human intoxication due to benthic dinoflagellates is ciguatera fish poisoning (Hallegraeff 1993, Lehane and Lewis 2000, Godhe et al. 2002, Gilbert et al. 2005). Benthic epiphytic dinoflagellates are known to be present in tropical and subtropical regions of the Pacific Ocean, Indian Ocean, and the Caribbean where they are found associated with seagrasses, green, brown, and red algae, as well as dead coral and sediment (Fukuyo 1981, Morton and Faust 1997, Turquet et al. 1998, Pearce et al. 2001, Aligizaki et al. 2008). However, some species also live in temperate regions (Pistocchi et al. 2011, Selina and Levchenko 2011). Many benthic flagellates are abundant in the intertidal substrata and their contributions to benthic and shallow marine ecosystems may be significant. The occurrence of
epiphytic and benthic dinoflagellates in temperate waters has been reported as evidence of increasing water temperature (Rhodes 2011, Jeong et al. 2012a). Several benthic flagellates species of the genera
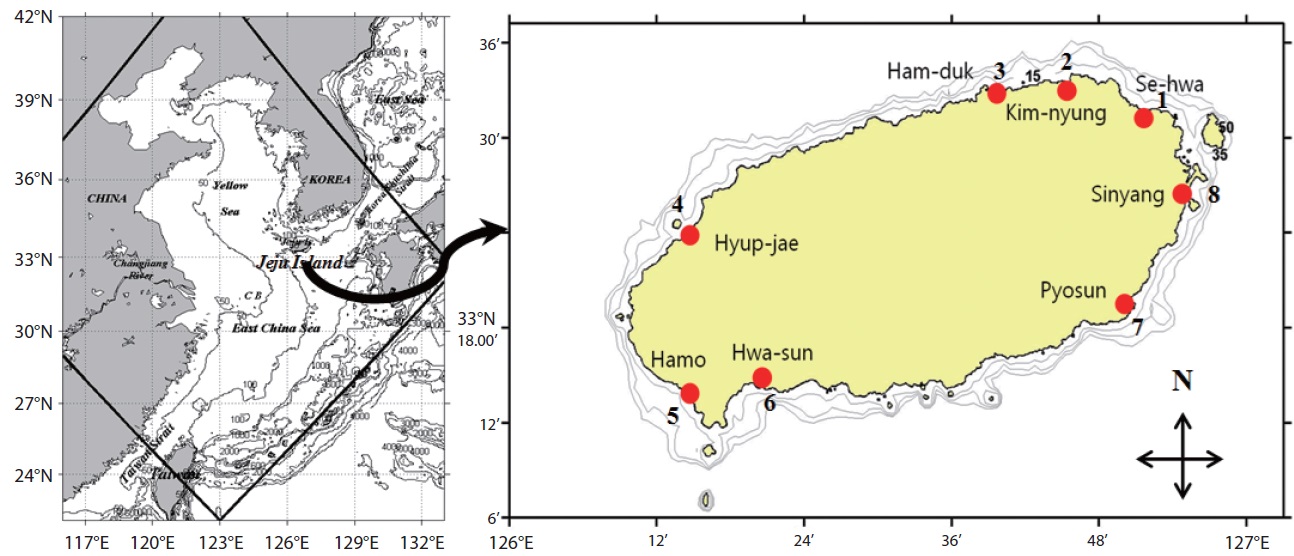
Jeju Island is a volcanic island located about 80 km south of the Korean peninsula in the southwestern sea of Korea with an area of about 1,830 km2. The coastline is mainly composed of rocky shore and sandy beaches with a few sand tidal flats. Jeju Island belongs to the temperate region classified based on air temperature. The Tsushima Warm Current (TWC), a branch of the Kuroshio Current with high water temperature and high salinity, strongly affects the adjacent sea of Jeju Island (Pang et al. 1996). Previously, the toxic dinoflagellates recorded from Korean temperate waters were planktonic but potentially toxic benthic sand dwelling and epiphytic species have not been well documented. Information on existing diversity and distribution patterns of benthic toxic/nontoxic dinoflagellates near Jeju Island is limited and sparse. To date, no toxic event caused by a marine benthic dinoflagellate has been reported from Jeju Island. Total 153 planktonic dinoflagellates were recorded from Korean waters by previous studies (Shim et al. 1981, Han and Yoo 1983a, 1983b, Yoo and Lee 1986, Lee et al. 1993, Shim 1994). Recently, Kim et al. (2011), Jeong et al. (2012a, 2012b), Kang et al. (2013) and Lim et al. (2013) described 6 benthic epiphytic dinoflagellates in the coastal waters of Jeju Island. However, in-depth information on taxonomy, distribution, diversity, and molecular phylogeny of benthic dinoflagellates from Korean waters has to be collected. Thus, the aim of this study was to document benthic dinoflagellates from the intertidal zone of Jeju Island coastal waters as part of a project called “Survey and Excavation of Korean Indigenous Species” of the National Institute of Biological Resources (NIBR) under the Ministry of Environment of Korea.
>
Study sites and sample collection
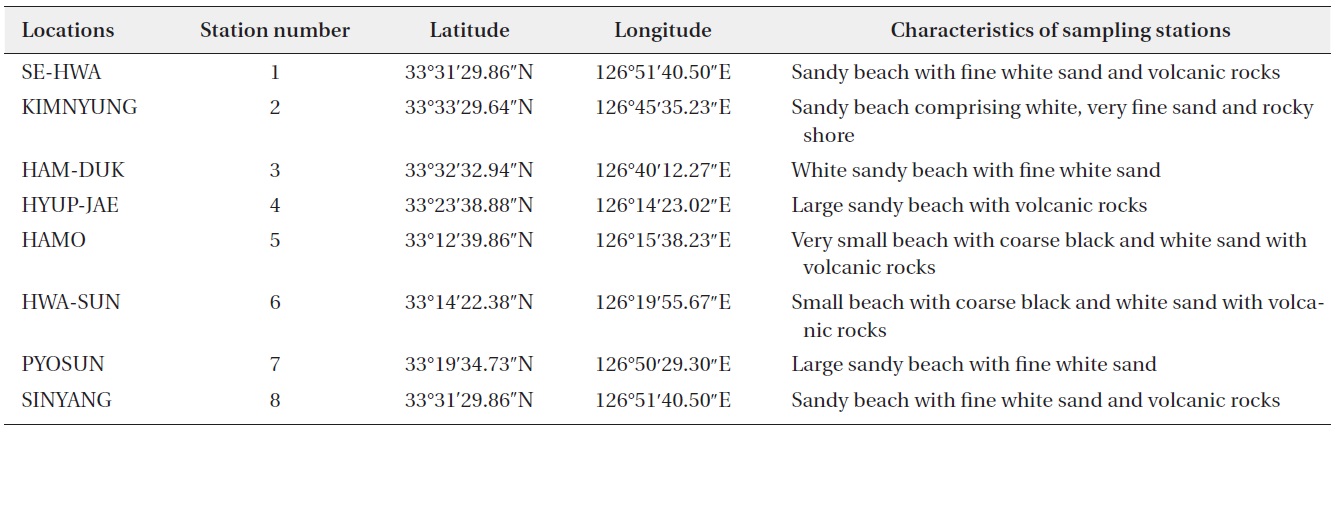
This study was carried out from March 2011 to February 2012 in the intertidal zone along the coasts of Jeju Island, Korea. Monthly sampling was carried out at eight designated stations (beaches) (Fig. 1) and 480 samples of sand sediment and macroalgae were collected during the lowest low tide. A brief description of the characteristics, latitudes, and longitudes of the sampling stations is presented in Table 1. Sand sediment samples (50–150 g wet weight) were collected from depths of 3–4 cm on sandy beaches using a plastic tube sample corer. The samples were transferred to plastic bottles with ambient seawater. In the laboratory, the sediment samples were vigorously shaken for 1 minute and the material was passed through 200 and 100 µm mesh sieves to remove large particles and finally passed through a 20 µm mesh sieve. The material retained by the sieve was resuspended in sterile filtered seawater in a Petri dish for live cell examination and isolation (modification of a method of Selina and Hoppenrath 2008).
To survey epiphytic dinoflagellates, we collected 20–100 g wet weight macroalgae (red, brown, and green) on the intertidal zone of the sampling stations by hand picking and placing them into separate plastic Ziploc bags with ambient seawater. Epiphytic dinoflagellates were separated from the macroalgae by vigorous shaking of the macroalgae into a plastic container with 200 ml of fresh filtered seawater for 1 minute to dislodge the attached dinoflagellate cells, and then we followed the procedure described above to extract the dinoflagellates from the sand sediment.
>
Dinoflagellate identification
Freshly-collected living dinoflagellates were isolated by the micropipette-washing method, placed on slide glass covered with a cover slip, and the morphometric features were observed under transmitted light with bright field and phase-contrast at ×400 magnification and photographed using a microscope (Axioplan 2; Carl Zeiss, Oberkochen, Germany) equipped with a digital camera (Axiocam ERc5s; Carl Zeiss). Both the dorsal and ventral sides of each dinoflagellate were examined. Cell size and some morphometric measurements were obtained from micrographs using Carl Zeiss ZEN Lite software (Carl Zeiss). Thecal plate patterns of armored dinoflagellates were identified using Calcofluor White M2R (Fluka, Buchs, Switzerland) (Fritz and Triemer 1985). The Calcofluor stained cells were examined using an epifluorescence (violet excitation at 430 nm, blue emission at 490 nm) microscope
Summary of sampling stations in the intertidal zone along the coasts of Jeju Island, Korea
(Axioplan 2; Carl Zeiss) equipped with a digital camera (Axiocam ICm 1; Carl Zeiss). Unarmored dinoflagellates were identified based on morphological features such as body contour and proportion, cingulum displacement, sulcus extension and direction on the epitheca, and presence and location of specific organelles. Dinoflagellates were identified using previously published schemes (Fukuyo 1981, Faust 1995, Horiguchi 1995, Faust 1996, Steidinger and Tangen 1997, Holmes 1998, Chinain et al. 1999, Faust et al. 1999, Hoppenrath 2000a, 2000b, Hansen et al. 2001, Faust and Gulledge 2002, Murray and Patterson 2002, Murray and Patterson 2004, Hoppenrath et al. 2007a, 2007b, Mohammad-Noor et al. 2007, Al-Yamani and Saburova 2010).
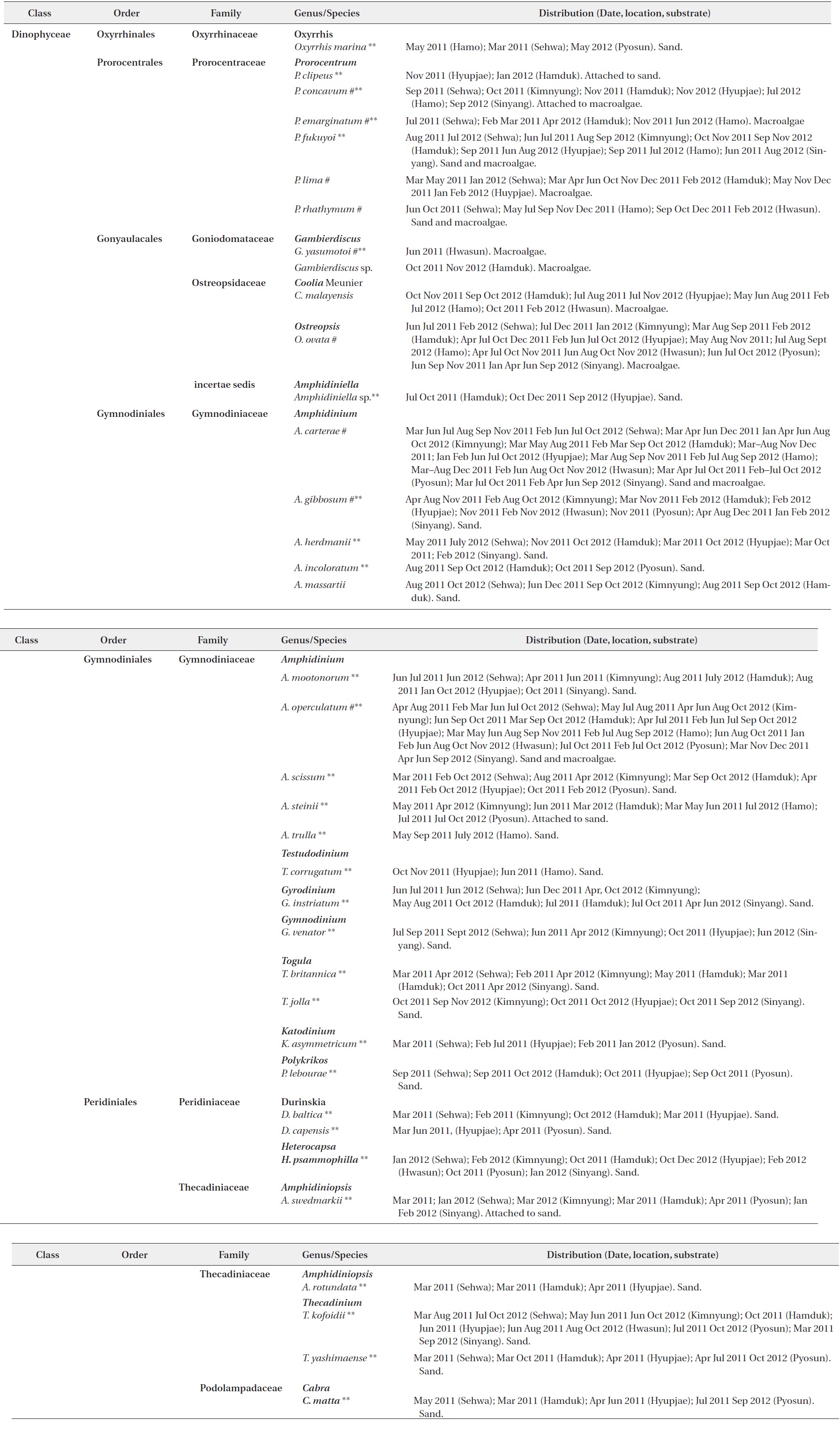
Thirty seven benthic dinoflagellate species belonging to 18 genera, including
[Table 2.] List of benthic dinoflagellate species observed from Jeju Island, Korea
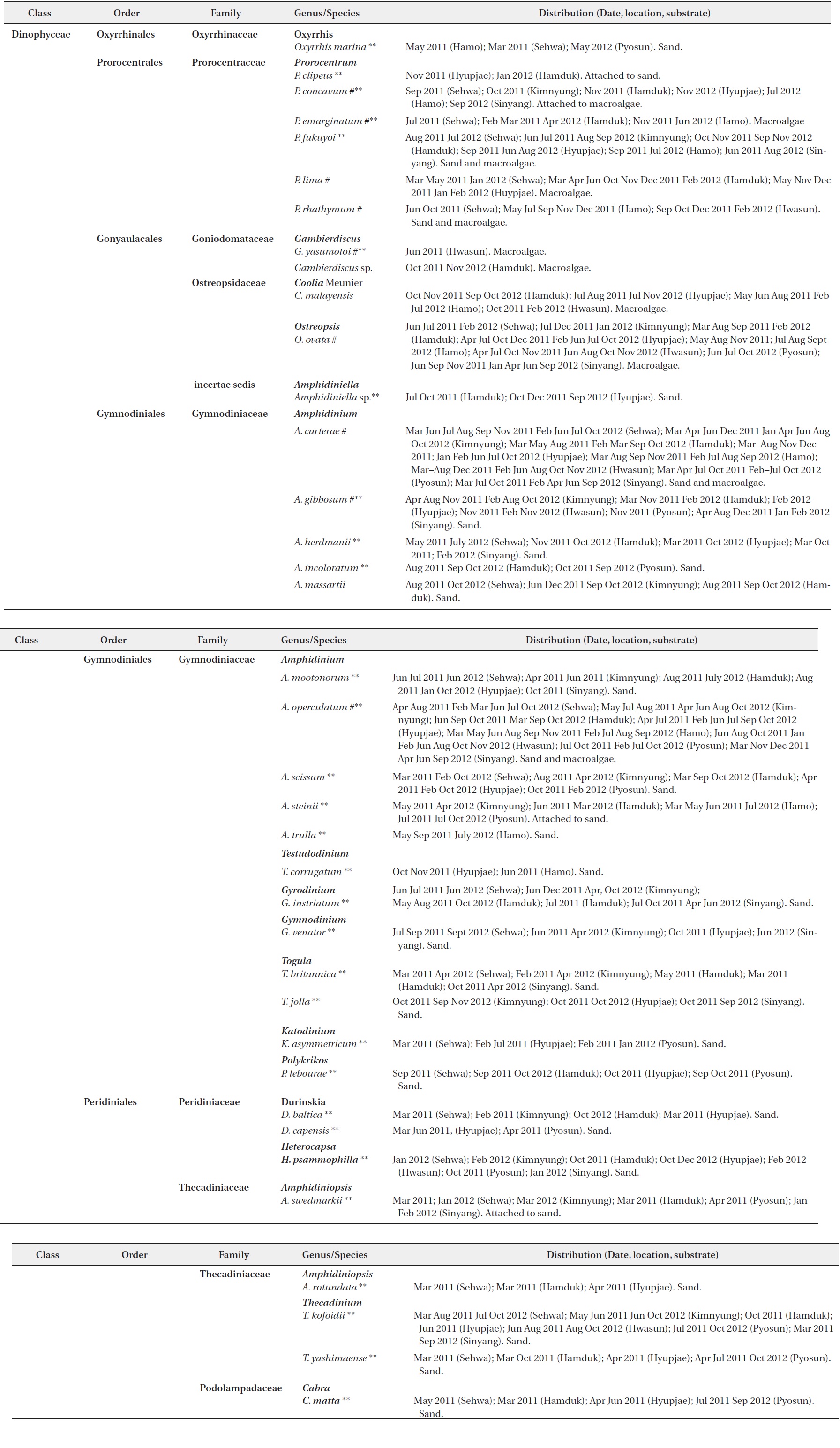
List of benthic dinoflagellate species observed from Jeju Island, Korea
viz.,
Of the 37 species identified in this study, 26 were recorded from only sand sediment, seven species were associated with macroalgae, and four species belonging to
Thirty taxa from the aforementioned benthic dinoflagellate genera constitute new records of benthic marine microflora in this study area and the systematic and distrubution are presented in Table 2. Among the identified species, 26 were recorded from only sand sediment, seven were associated with macroalgae and four were found in both sand and macroalgal samples (Table 2). Taxonomic information, illustrations, classification, references, basionyms, synonyms of the identified benthic dinoflagellates are described below.
>
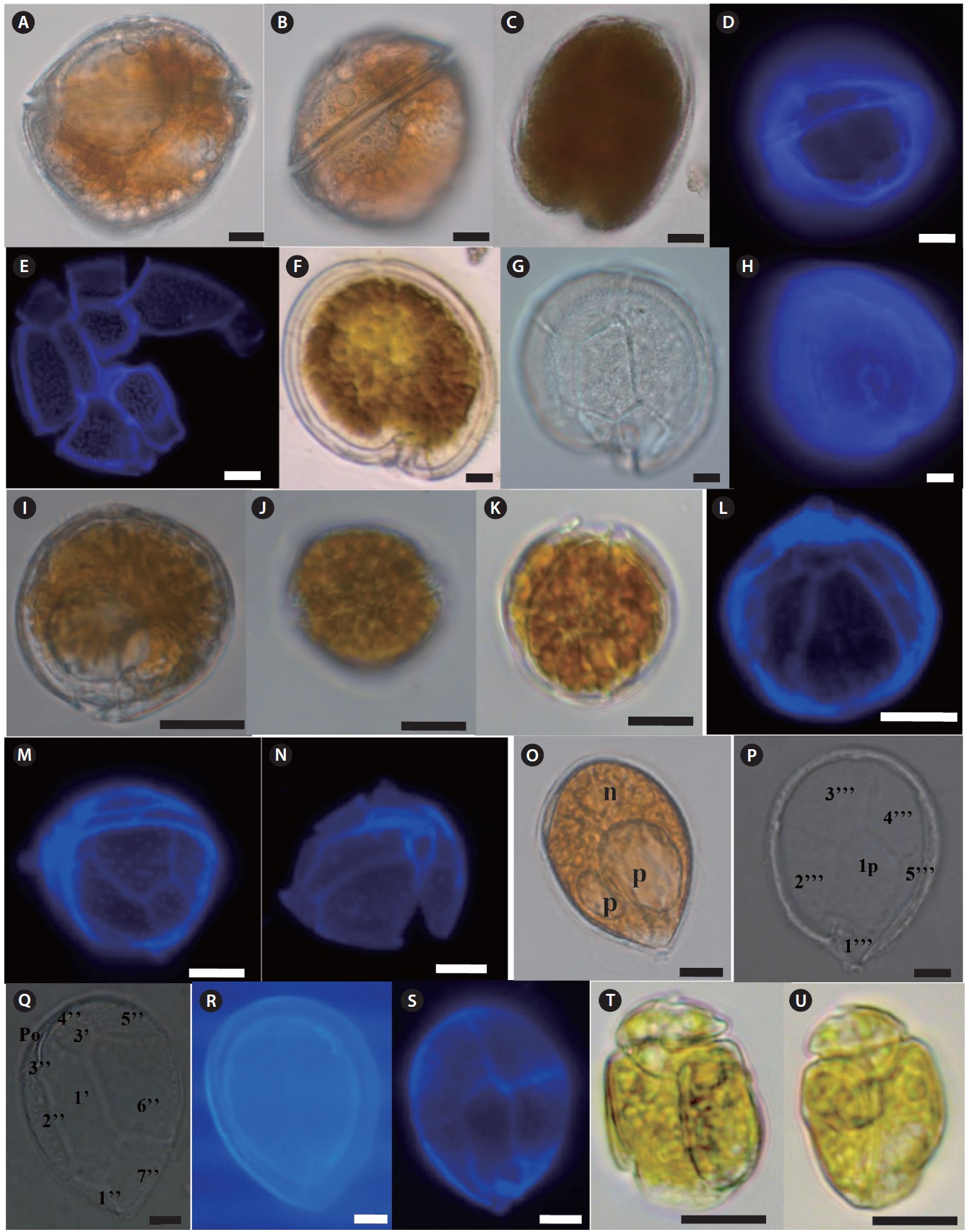
Genus Oxyrrhis Dujardin 1841
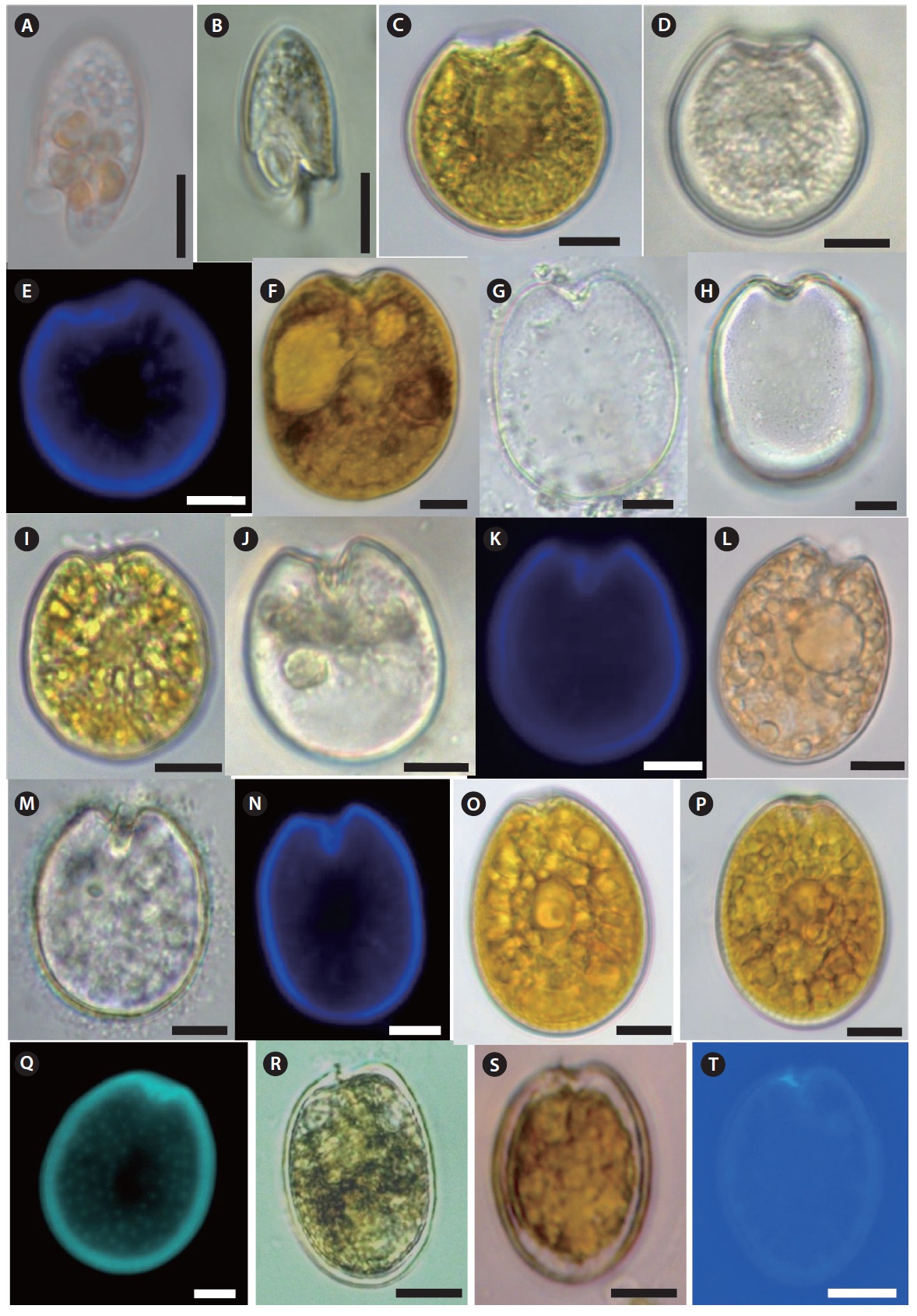
Oxyrrhis marina Dujardin 1841 (
Synonyms:
References: Dujardin 1841, p 347, pl. 5: 4; Kofoid and Swezy 1921, pp 117–119, fig. 3; Lebour 1925, p 19, pl. I: 6a–e; Dodge 1982, p 111, fig. 13E-F.
Specimen examined: Slide LJB2012-07 at the National Institute of Biological Resources (NIBR), Incheon.
Description: Cell elongated to oval shape. Anterior end broadly conical and posterior end is asymmetrical. Posterior end of cell contains a tentacular lobe. Transverse and longitudinal flagella originate to the left and right of the lobe, respectively. Food vacuoles are observed in the cytoplasm. The nucleus is located near the anterior end of cell. The cell is unarmored, nonphotosynthetic, colorless or slightly pinkish.
Size: Length (L) (µm): 12–30 (23.7 ± 3.4, mean ± standard deviation); Width (W) (µm): 10–28 (15.5 ± 2.8)
>
Genus Prorocentrum Ehrenberg 1833
Prorocentrum clipeus Hoppenrath 2000 (
References: Hoppenrath 2000a, pp 30–31, figs. 1–12.
Specimen examined: Slide LJB2012-04 at the NIBR, Incheon.
Description: Armored photosynthetic dinoflagellate. The cell is nearly round. Cell contains yellow–brown chloroplasts, a pusule, a small apical spine, and a central pyrenoid. The periflagellar region is wide-arc shape. The large kidney-shaped nucleus is located posteriorly.
Size: L (µm): 30–35 (33.8 ± 0.6); W (µm): 32–36.5 (34.0 ± 0.4).
Prorocentrum concavum Fukuyo 1981 (
Synonym:
References: Fukuyo 1981, pp 968–969, figs. 13–19, 49; Faust et al. 1999, p 4, fig. 2a–g; Mohammad-Noor et al. 2007, pp 635–639, figs. 3a–j, 14a–c.
Specimen examined: Slide LJB2012-05 at the NIBR, Incheon.
Description: Armored phototsynthetic dinoflagellate with golden-brown chloroplasts. The cell is broadly ovoidal in the valve view, widest behind the middle. Two cup-shaped pyrenoids are present at the anterior center and just beneath the valves. The anterior margin is concave. Both valves have fine depressions covering the whole surface. Many trichocyst pores scattered over the valve except the central area, but are denser near the margin.
Size: L (µm): 45.4–51.3 (44.1 ± 1.2); W (µm): 38–48.7 (39.6 ± 1.7).
Harmful effects: Produces three diol esters of okadaic acid (Hu et al. 1993) and ichthyotoxin (Yasumoto et al.1987).
Prorocentrum emarginatum Fukuyo 1981 (
References: Fukuyo 1981, p 968, figs. 8–12, 48; Faust 1990, p 549, figs. 1–4, 29; Hansen et al. 2001, pp 38–39, pl. 3G, 3H, 6E; Larsen and Nguyen 2004, p 58, pl. 4: 1–3; Mohammad-Noor et al. 2007, pp 639–640, figs. 4a–j, 15a–b.
Specimen examined: Slide LJB2011-15 at the NIBR, Incheon.
Description: Armored photosynthetic dinoflagellate with golden-brown chloroplasts, central pyrenoid, and posterior nucleus. Cells are broadly oval to rotundate. Valve surface is smooth. The periflagellar area is deep and V-shaped and contains a wing like structure. The right valve has an inclined, rectangular flagellar structure and the left valve is concave and deeply indented. Both valves are concave at the center, and the anterior end is deeply indented.
Size: L (µm): 35–40 (30.35 ± 0.4); W (µm): 28–34 (26.75 ± 0.8).
Harmful effects: Potentially toxic species (Hansen et al. 2001). Turquet (1997) found low hemolytic and fibroblast activity in a crude extract of cells from the southwestern Indian Ocean.
Prorocentrum fukuyoi Murray and Nagahama 2007 (
Reference: Murray et al. 2007, pp 93–95, figs. 1–13.
Specimen examined: Slide LJB2011-16 at the NIBR, Incheon.
Description: Armored photosynthetic dinoflagellate. Cells are narrow oval to almost oblong shape. The apical area is narrow, slightly curved, V-shaped, and a spine is present. Valve surface is smooth. Rows of large size pores radiate towards the center. There is a round pusule near the flagella origin. Large plastids radiate toward the periphery of the cell. There is a pyrenoid with a ring-shaped starch-sheath.
Size: L (µm): 30–42 (37.1 ± 1.9); W (µm): 25–32 (31.20 ± 0.9).
Prorocentrum lima (Ehrenberg) Dodge 1975 (
Synonyms:
References: Dodge 1982, p 30, fig. 2G-H; Hansen et al. 2001, p 40, pl. 5: A–D; Larsen and Nguyen 2004, p 59, pl. 5: 1–5.
Description: Armored photosynthetic dinoflagellate with golden-brown chloroplasts, a prominent central pyrenoid, and a posterior nucleus. Cells are oval to oblong. The cell margin is round at the posterior end and flat at the anterior end. The periflagellar area is small with a V-shaped depression. The thecal surface is smooth with scattered pores that are absent in the center. Marginal pores are present. Both valves are concave.
Size: L (µm): 34.5–43.3 (41.8 ± 1.3); W (µm): 27–33 (31.2 ± 1.5).
Harmful effects: DSP producer (Prokic et al. 1998, Sechet et al. 1998) and produces okadaic acid and dinophysistoxin (Marr et al. 1992, Barbier et al. 1999).
Prorocentrum rhathymum Loeblich III, Sherley et Schmidt 1979 (
References: Loeblich et al. 1979, pp 115–116, 118–119, figs. 8–13; Fukuyo 1981, p 968, figs. 5–7, 47; Cortés-Altamirano and Sierra-Beltrán 2003, pp 223–224, figs. 1o–q, 3; Mohammad-Noor et al. 2007, pp 652–653, figs. 10a–i, 21a-b.
Specimen examined: Slide LJB2012-06 at the NIBR, Incheon.
Description: Armored photosynthetic dinoflagellate. Cell is broadly oval. The left valve has an indentation at the anterior end. A small spine is located at the ventral side of the auxillary pore. The nucleus is located in the posterior of the cell, and the pyrenoid is central. The thecal surface is smooth, and two different pore sizes are present. The larger pores are radially arranged and perpendicular to the valve margin.
Size: L (µm): 32.5–40.3 (34.5 ± 1.3); W (µm):20–25 (22.7 ± 0.8).
Harmful effects: Produce toxins with hemolytic activity (Nakajima et al. 1981), and water-soluble fast-acting toxins (Tindall et al. 1989).
>
Genus Gambierdiscus Adachi and Fukuyo 1979
Gambierdiscus yasumotoi Holmes 1998 (
References: Holmes 1998, pp 663–664, figs. 1–8; Hansen et al. 2001, p 55, pl. 9: D–F.
Specimen examined: Slide LJB2011-01 at the NIBR, Incheon.
Description: Armored photosynthetic dinoflagellate. Cells are globular in general. Cells are circular to ovoid in apical view. The thecal surface is smooth and covered with numerous small pores. The cingulum is median to premedian, deep, and narrow. Sulcus is broad and indented. The plate formula: po, 3’7’’, 6c, 6s, 5’’’, 1p, 2’’’’. The apical pore plate (po) has a long fish-hook-shaped pore. Numerous small golden-brown chloroplasts are present. Cell contains 2–3 large hyaline pusules centrally located or in the hypocone.
Size: L (µm): 56–67.5 (61.60 ± 3.2); W (µm): 46–58.5 (52 ± 2.6).
Harmful effects:
Gambierdiscus sp. (
Description: Cells are large, rounded obliquely ellipsoidal in apical view, lenticular in ventral view, anterio-posteriorly compressed. Epitheca and hypotheca are almost equal in size. The cingulum is deep, narrow, and ascending, and the sulcus is deep and short. The thecal surface is smooth and covered with numerous small pores.
Size: L (µm): 69.5–81 (77.0 ± 3.2); W (µm): 76.5–87.2 (79.1 ± 2.6).
Coolia malayensis?Leaw, P.-T.Lim et Usup 2010 (
References: Leaw et al. 2010, pp 164–165, figs. 2–4.
Specimen examined: Slide LJB2011-09 at the NIBR, Incheon.
Description: Armored photosynthetic dinoflagellate. Cells are small, round in dorsoventral and lateral views. Thecal surface is smooth, irregularly scattered with large pores. The inner part of the thecal pores has scattered, very fine perforations. The plate formula: po, 3’, 7’’, 6c, 6s (?), 5’’’, 2’’’’.
Size: L (µm): 26.5–35.0 (30.5 ± 3.2); W (µm): 25–33.5 (28.3 ± 2.6).
>
Genus Ostreopsis Schmidt 1901
Ostreopsis ovata Fukuyo 1981 (
References: Fukuyo 1981, p 971, figs. 35–38, 54, 55; Faust et al. 1996, pp 1057–1059, figs. 16–23; Larsen and Nguyen 2004, pp 115–117, pl. 20: 1–6, pl. 21: 5; Penna et al. 2005, pp 216–217, fig. 4; Aligizaki and Nikolaidis 2006, pp 719–722, fig. 2.
Specimen examined: Slide LJB2011-10 at the NIBR, Incheon.
Description: Armored photosynthetic dinoflagellate with many golden chloroplasts. Cells are tear-shaped, ovoid to oblong, pointed toward the ventral area. Epitheca and hypotheca about equal in size.The plate formula is: Po, 3’, 7’’, 6c, 6?s, 5’’’, 2’’’’, 1p, The thecal plates are thin and delicate. The thecal surface is smooth, ornamented with minute, evenly scattered pores. Nucleus is located at the posterior end of the cell. One or two pussels observed in the dorsal part of the cell.
Size: L (µm): 30–64 (51.46 ± 2.1); W (µm): 20–58 (34.5 ± 1.7).
Harmful effects: Produces a toxic butanol-soluble compound (Elbrächter and Faust 2002) related to human health problems in tourists (Aligizaki and Nikolaidis 2006).
>
Genus Amphidiniella Horiguchi 1995
Amphidiniella sp. (
Reference: Murray 2003, p 83, fig. 2. 26, f–j, l, m.
Specimen examined: Slide LJB2012-17 at the NIBR, Incheon.
Description: Armored photosynthetic dinoflagellate with yellow-brown chloroplast, posterior nucleus, and central pyrenoid. Cell dorsoventrally flattened and oval from the ventral side. Epicone is small, asymmetric, deflected to the left and contain a hook-like projection. Hypocone is large with roundish posterior end. The cingulum is wide, left-handed. Sulcus is wide, with a prominent right ridge, reaches the antapex.
Size: L (µm): 22–30 (25.1 ± 0.8); W (µm): 18–25 (19.8 ± 0.7).
>
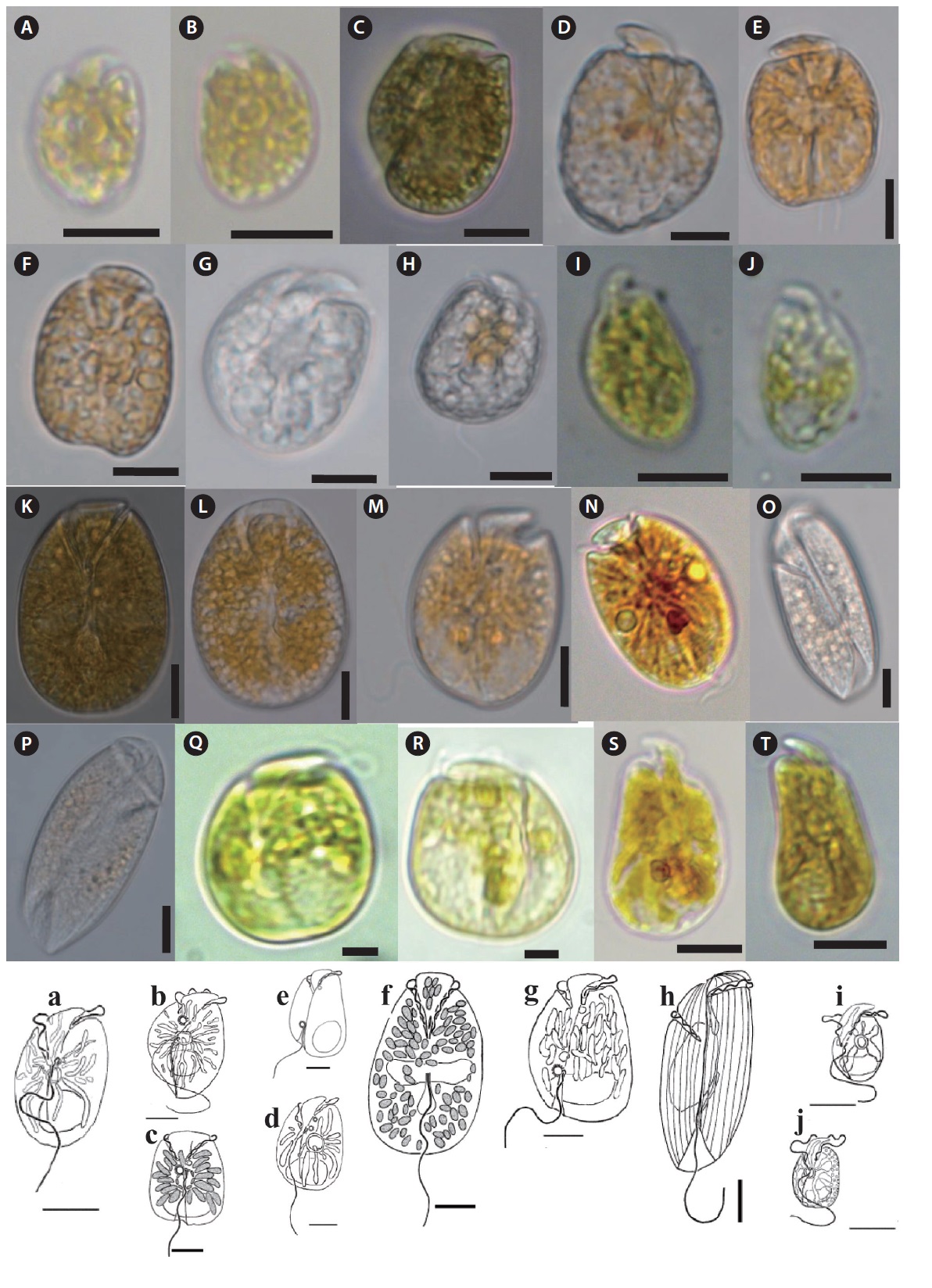
Genus Amphidinium Claparede and Lachmann 1859
Amphidinium carterae Hulburt 1957 (
Synonyms:
References: Hulburt 1957, p 216, pl. 1: 1; Dodge 1982, p 69, fig. 7J; Larsen and Patterson 1990, p 889, fig. 47d; Murray and Patterson 2002, p. 284, figs. 11–15, 75; Flø Jørgensen et al. 2004b, p 353, fig. 1A; Murray et al. 2004, p 376, figs. 2F, 3E, 8A–F.
Description: Unarmored photosynthetic dinoflagellate with yellow-brown single chloroplast, posterior rounded nucleus, and central ring like starch sheathed pyrenoid. Cell is dorso-ventrally flattened and oblong to oval in ventral view. Eepicone is crescent shaped, deflected to the left, and minute compared to the hypocone. Line drawing of this species is shown in Fig. 4a (Murray and Patterson 2002).
Size: L (µm): 14.5–21.8 (17.2 ± 0.8); W (µm): 9.0–13.5 (11.8 ± 0.7).
Harmful effects: Hemolysin compounds have been isolated from this species and ichthyotoxicity is known (Yasumoto et al. 1987).
Amphidinium gibbosum (Maranda and Shimizu 1996) Flø Jørgensen and Murray 2004 (
Basionym:
Synonym:
References: Maranda and Shimizu 1996, pp 873–879; Murray et al. 2004, p 373, figs. 2B, 3B, 5A–D.
Specimen examined: Slide LJB2012-01 at the NIBR, Incheon.
Description: Unarmored photosynthetic dinoflagellate with posterior rounded nucleus and large ring like pyrenoid located at the center of the hypocone. Cells are asymmetrical, ellipsoid, and dorsoventrally flattened. The hypocone is oval to heart shaped. The posterior part of the right side has a ‘‘hump-backed’’appearance. Line drawing of this species is shown in Fig. 4b (Murray et al. 2004).
Size: L (µm): 28–38 (32 ± 0.55); W (µm):17–22 (19.8 ± 0.6).
Harmful effects: Produces cytotoxic metabolites, the most potent showing antitumour activity (Maranda and Shimizu, 1996).
Amphidinium herdmanii Kofoid and Swezy 1921 (
Synonym:
References: Lebour 1925, T. 2, p 23, fig. 2; Schiller 1933, p 294, fig. 238a–f; Dodge 1982, p 70, fig. 7H; Larsen 1985, p 21, figs. 20–25, 91; Murray and Patterson 2002, p 286, figs. 22–25, 79; Flø Jørgensen et al. 2004b: p 353, fig. 1C.
Specimen examined: Slide LJB2011-03 at the NIBR, Incheon.
Description: Unarmored photosynthetic dinoflagellate with posterior cresent shaped nucleus and pyrenoid like structure from where elongated chloroplast radiates. Cells are quadrangular-broadly oblong shape and dorsoventrally compressed. Epicone is triangular, slightly asymmetrical around the longitudinal axis, deflected toward the left. Hypocone is slightly asymmetrical with the left handside being a little longer than the right. Line drawing of this species is shown in Fig. 4c (Murray and Patterson 2002).
Size: L (µm): 28–34 (30 ± 0.9); W (µm):18–24 (21.8 ± 0.8).
Amphidinium incoloratum Campbell 1973 (
Synonym:
References: Larsen and Patterson 1990, p 890, figs. 43c-d, 44c; Murray and Patterson 2002, p 286, figs. 32–35, 80; Flø Jørgensen et al. 2004b, p 353, fig. 1B.
Specimen examined: Slide LJB2012-06 at the NIBR, Incheon.
Description: Unarmored nonphotosynthetic dinoflagellate with posterior rounded nucleus and colorless lipid globules or food particles in cytoplasm. Cells are broadly ellipsoidal to oval from the ventral side, with a relatively straight left side and a convex right side. The epicone is deflected to the left. The cingulum and sulcus are asymmetrical.
The longitudinal flagellum arises in a pocket to the left and just below the start of the sulcus. Line drawing of this species is shown in Fig. 4d (Murray and Patterson 2002).
Size: L (µm): 28–36 (31.6 ± 1.5); W (µm):16–25 (21 ± 1.8).
Amphidinium massartii Biecheler 1952 (
Synonyms:
References: Biecheler 1952, p 24, figs 4, 5; Murray et al. 2004, p 375, figs. 2E, 3F, 7A–E; Lee et al. 2013, p 223, fig 6.
Specimen examined: Slide LJB2012-04 at the NIBR, Incheon.
Description: Unarmored photosynthetic dinoflagellate with rounded or crescent-shaped nucleus in the posterior part of the hypocone, central ringlike starch-sheathed pyrenoid. Cells are dorsoventrally flattened and oval from the ventral side. Single yellow-green plastid with several narrow lobes radiating out from the center of the cell. The epicone is minute, crescent shaped, and pointing toward the left. The hypocone is slightly pointed at the antapex. Line drawing of this species is shown in Fig. 4e (Murray et al. 2004).
Size: L (µm): 13–21 (16.6 ± 2.1); W (µm): 9–14.8 (10.4 ± 1.9).
Amphidinium mootonorum Murray and Patterson 2002 (
Reference: Murray and Patterson 2002, p 289, figs. 40–42, 83.
Specimen examined: Slide LJB2011-04 at the NIBR, Incheon.
Description: Unarmored photosynthetic dinoflagellate with many small, yellow-brown chloroplasts and an elongate oval nucleus located in the center of the hypocone. Cells are dorsoventrally flattened and oval in ventral side. Epicone is “stem-shaped” at the junction of the cingulum and sulcus. Epicone slightly deflected towards the left. Cingulum is relatively wide. Sulcus narrow initially, opens into a teardrop-shaped indentation. Line drawing of this species is shown in Fig. 4f (Murray and Patterson 2002).
Size: L (µm): 38–52 (44 ± 2.4); W (µm): 28–42 (34.5 ± 2.8).
Amphidinium operculatum Claparede and Lachmann 1859 (
Synonyms:
References: Claparède and Lachmann 1859, p 410, pl. 20: 9–10; Lebour 1925, T. 2, p 22, fig. 8a; Schiller 1933, p 304, fig. 304; Dodge 1982, p 73, fig. 7I; Flø Jørgensen et al. 2004b, p 358, fig. 2; Larsen and Nguyen 2004, p 119, pl. 22: 2; Murray et al. 2004, p 368, figs. 1A–F, 2A, 3A.
Specimen examined: Slide LJB2012-03 at the NIBR, Incheon.
Description: Unarmored photosynthetic dinoflagellate with oval or crescent-shaped nucleus in the posterior part of the hypocone. The multiple plastids are yellow-brown and elongated, appearing to radiate to the cell periphery. Cell is ovoid to ellipsoidal and dorsoventrally flattened. The right side of the hypocone is convex but left is almost straight. A small epicone is irregularly triangular with the anterior left tip deflected to the left. The cingulum is displaced and slightly descending. The sulcus originates in the lower one-third of the cell. Line drawing of this species is shown in Fig. 4g (Murray et al. 2004).
Size: L (µm): 20.3–48 (33.6 ± 2.5); W (µm): 16–26.8 (22.4 ± 2.9).
Harmful effects: Compounds with hemolytic and antifungal properties (amphidinols) are reported, and they may be toxic to fish (Yasumoto et al. 1987).
Amphidinium scissum Kofoid and Swezy 1921 (
Synonym:
References: Kofoid and Swezy 1921, p 150, pl. 2: 22 U1; Dodge 1982, p 67, Fig. 6f; Murray and Patterson 2002, p 293, figs. 56–59, 87; Flø Jørgensen et al. 2004b, p 353, fig. 1J.
Specimen examined: Slide LJB2011-05 at the NIBR, Incheon.
Description: Unarmored non-photosynthetic dinoflagellate with ellipsoidal nucleus in the central-right side of the hypocone. Cells are elongate ellipsoidal to oblong from the ventral side, dorsoventrally flattened. The epicone is asymmetrical, and slopes down towards the right. The cingulum is relatively deep. The sulcus is narrow and shallow, reaches to the posterior end. Line drawing of this species is shown in Fig. 4h (Murray and Patterson 2002).
Size: L (µm): 48–56 (50 ± 0.6); W (µm): 20–24 (21 ± 0.4).
Amphidinium steinii Lemmermann 1910 (
Synonyms:
References: Lebour 1925, T. 2, p 23, fig. 8b; Schiller 1933, p 316, figs. 310a, b; Murray et al. 2004, p 371, figs. 2C, 3D, 4A–J.
Specimen examined: Slide LJB2012-02 at the NIBR, Incheon.
Description: Unarmored phototrophic dinoflagellate with round to oval nucleus in the posterior part of the hypocone, single yellow-brown plastid and the ringlike starch-sheathed pyrenoid. Cells are oval from the ventral side and dorsoventrally flattened. The epicone is minute, triangular, curved anteriorly, and clearly deflected to the left. The sulcus begins just to the right of the mid-ventral line. Two small pusules are present, one below the origin of the cingulum and the other to the right of the origin of the sulcus. Line drawing of this species is shown in Fig. 4i (Murray et al. 2004).
Size: L (µm): 25.3–32.7 (28.5 ± 1.0); W (µm): 18–24 (20.4 ± 0.9).
Amphidinium trulla Murray, Rhodes et Flø Jø rgensen 2004 (
References: Murray et al. 2004, p 374, figs. 2D, 3C, 6A–D.
Specimen examined: Slide LJB2012-05 at the NIBR, Incheon.
Description: Unarmored non-photosynthetic dinoflagellate with ellipsoidal nucleus in the central-right side of the hypocone, yellow-brown plastid, and central pyrenoid. Cells are oval from the ventral side and dorsoventrally flattened. The minute crescent-shaped epicone overlays the anterior part of the hypocone. The epicone is anteriorly curved rather than flat and slopes to the right. Line drawing of this species is shown in Fig. 4j (Murray and Patterson 2002).
Size: L (µm): 18–31 (26.5 ± 2.7); W (µm): 13–24 (14.9 ± 2.5).
>
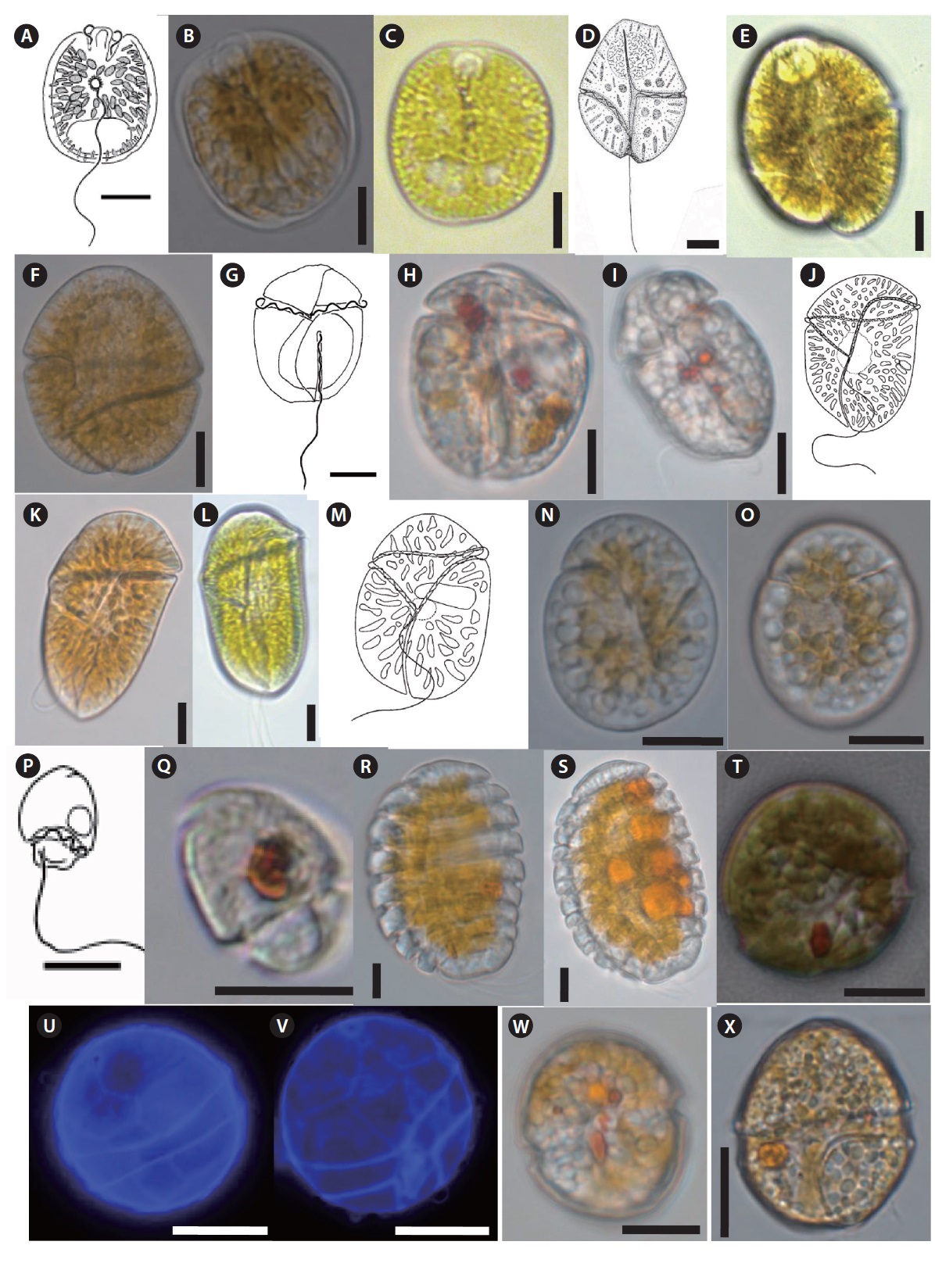
Genus Testudodinium (Larsen and Patterson) Horiguchi, Tamura, et Yamaguchi 2012
Testudodinium corrugatum (Larsen and Patterson) Horiguchi, Tamura, et Yamaguchi 2012 (
Basyonym:
References: Larsen and Patterson 1990, p 889, figs. 44a, 45a, b; Murray and Patterson 2002, p 284, figs. 16–18, 76, 77; Horiguchi et al. 2012, pp 143–145, figs 18–31.
Specimen examined: Slide LJB2011-02 at the NIBR, Incheon.
Description: Unarmored photosynthetic dinoflagellate with yellow-brown chloroplasts and oval to crescent-shaped posterior nucleus. Cells are dorsoventrally flattened and oval from the ventral side. Longitudinal flagellum originates from just below the pusule. Epicone small, club-shaped, hypocone forms a “collar” around the epicone. Line drawing of this species is shown in Fig. 5A (Murray and Patterson 2004).
Size: L (µm): 26–32 (28.2 ± 0.8); W (µm): 21–28 (24 ± 0.7).
>
Genus Gyrodinium Kofoid and Swezy 1921
Gyrodinium instriatum Freudenthal and Lee 1963 (
References: Freudenthal and Lee 1963, figs. 15–17; Steidinger and Tangen 1997, p 450, pl. 1, 19.
Specimen examined: Slide LJB2012-03 at the NIBR, Incheon.
Description: Unarmored photosynthetic dinoflagellate with large elliptical nucleus in the epicone. Cells are ovoid and slightly dorsoventrally compressed. Epicone tapering gently toward truncate apex in ventral and dorsal views. Hypocone is slightly longer and wider, slightly more truncate at antapex, more trapezoidal in side view. Cingulum is narrow. Sulcus is invading spherical region of the epicone and extending posteriorly to the antapex. Line drawing of this species is shown in Fig. 5D (Freudenthal and Lee 1963).
Size: L (µm): 48–68 (56.2 ± 3.5); W (µm): 32–56 (44.9 ± 2.5).
>
Genus Gymnodinium Claparede and Lachmann 1859
Gymnodinium venator Flø Jørgensen and Murray 2004 (
Basionym:
Synonym:
References: Herdman 1922, pp 27–28, pl. 1: 7; Dodge 1982, p 67, fig. 6G; Murray and Patterson 2002, p 291, figs. 47–51, 85; Flø Jørgensen et al. 2004b, p 353, fig. 1H; Flø Jørgensen et al. 2004b, p 1181.
Specimen examined: Slide LJB2012-08 at the NIBR, Incheon.
Description: Unarmored non-photosynthetic dinoflagellate with crescent-shaped nucleus in the upper part of the cell, and colored bodies in the posterior end of the hypocone. Cell is oblong shape, dorsoventrally flattened. Epicone is obtuse, hypocone broadly sack-shaped. Cingulum is wide, incised, tapering distally, descending in a left turning spiral, slightly displaced. Sulcus extends onto the epicone reaching the apex to produce a small notch, acute on the epicone. Line drawing of this species is shown in Fig. 5G (Murray and Patterson 2004).
Size: L (µm): 28–39 (36.8 ± 0.9); W (µm): 25–32.5 (29.0 ± 0.8).
>
Genus Togula Flø Jørgensen, Murray et Daugbjerg 2004
Togula britannica (Herdman 1922) Flø Jørgensen, Murray et Daugbjerg 2004 (
Basionym:
References: Herdman 1922, p 18, fig. 5; Lebour 1925, p 22, fig. 8a; Dodge 1982, p 68, fig. 7E; Larsen 1985, p 20, figs. 14–19, 90; Flø Jørgensen et al. 2004a, pp 289–291, figs. 2–12, 36.
Specimen examined: Slide LJB2011-07 at the NIBR, Incheon.
Description: Unarmored non-photosynthetic dinoflagellate with nucleus in the center of the cell, and irregular golden-brown chloroplasts. Cell is elongate ellipsoidal, dorsoventrally flattened. The longitudinal flagellum length is almost the same as the cell length. The cingulum is highly asymmetrical. In ventral view, the proximal end originates slightly right of the center of the cell and has a straight longitudinal to left course towards the apex. Line drawing of this species is shown in Fig. 5J (Flø Jørgensen et al. 2004a).
Size: L (µm): 40–68 (50.2 ± 3.2); W (µm): 30–40 (34.8 ± 2.6).
Togula jolla Flø Jørgensen, Murray et Daugbjerg 2004 (
References: Flø Jørgensen et al. 2004a, pp 295-295, figs. 22–35.
Specimen examined: Slide LJB2012-09 at the NIBR, Incheon.
Size: L (µm): 24–40 (32.4 ± 3.9); W (µm): 20–33 (26.0 ± 2.8).
Katodinium asymmetricum (Massart 1920) Loeblich 1965 (
Synonyms:
References: Loeblich 1965, p 16; Dodge 1982, p 127, fig. 15D; Larsen and Patterson 1990, p 895, fig. 44f; Al-Qassab et al. 2002, p 118, fig.12p.
Specimen examined: Slide LJB2011-06 at the NIBR, Incheon.
Description: Armored non-photosynthetic dinoflagellate. Cell is oblong and dorsoventrally flattened. Epicone dome-shaped and its length is about two-thirds of the total cell length. The outline of the epicone is broken by a small incision a little to the left of the mid-ventral line. Hypocone is narrower than the epicone. Line drawing of this species is shown in Fig. 5P (Al-Qassab et al. 2002).
Size: L (µm): 11–18 (14 ± 0.5); W (µm): 8–12 (10 ± 0.2).
>
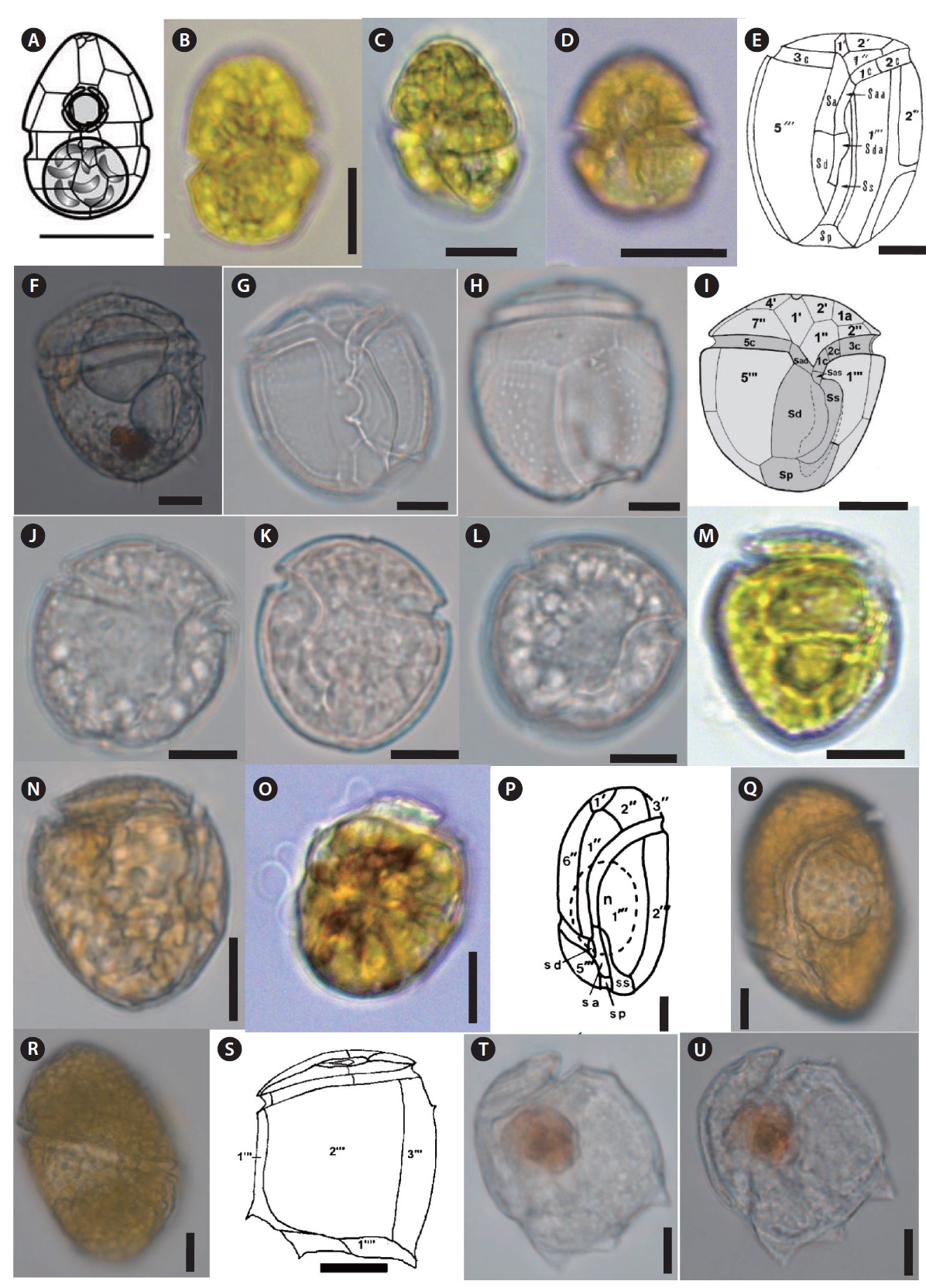
Genus Polykrikos Butschli 1873
Polykrikos lebourae Herdman 1923 emend. Hoppenrath et Leander 2007(
Reference: Hoppenrath and Leander 2007, p 370, fig.1e,1f.
Specimen examined: Slide LJB2012-16 at the NIBR, Incheon.
Description: Unarmored non-photosynthetic dinoflagellate with pseudocolonies consisting of eight zooids, eight sets of longitudinal and transverse flagella, and two nuclei; small, golden-brown, spherical, or spindle-shaped plastids with central pyrenoid. Pseudocolonies are oval shape and obliquely flattened. Loop-shaped acrobase presents. Sometimes the cell contains food bodies. Fused sulci and cinguli descending displaced about one to two cingular widths.
Size: L (µm): 40–84.5 (58.2 ± 3.9); W (µm): 20–56 (37.8 ± 2.8).
>
Genus Durinskia Carty and Cox 1986
Durinskia baltica Carty and Cox 1986 (
Reference: Carty and Cox 1986, p 200, figs. 7, 8, 9b, 10b, 11–14.
Specimen examined: Slide LJB2011-12 at the NIBR, Incheon.
Description: Armored photosynthetic dinoflagellate with numerous golden-brown chloroplasts. Cells round to ovoid, slightly dorsi-ventraly flattened, small in size. Epitheca is slightly larger than hypotheca. Cingulum well excavated, left handed, and displaced; sulcus conspicuous. A conspicuous, bright red, large, and rectangular eyespot is located on the left part of sulcus. The thecal plate formula is Po, x, 4’, 2a, 6’’, 5c, 4s, 5’’’, 2’’’’.
Size: L (µm): 16–28 (20.2 ± 2.4); W (µm): 15.8–25 (18.8 ± 2.8).
Durinskia capensis Pienaar, Sakai et Horiguchi 2007 (
Reference: Pienaar et al. 2007, pp 249–253, figs. 1–7.
Specimen examined: Slide LJB2011-13 at the NIBR, Incheon.
Description: Armored photosynthetic dinoflagellate. Cell rounded to ovoidal and dorsoventrally flattened. Hemispherical epitheca isslightly larger than the hemispherical trapezoidal hypotheca. Cingulum well excavated, left-handed. Sulcus is conspicuous. The hook-shaped eyespot is conspicuous, bright red, and located on left side of the upper part of the sulcus.
Size: L (µm): 22–28 (24.5 ± 0.3); W (µm): 18–25 (22.8 ± 0.8).
>
Genus Heterocapsa Stein 1883
Heterocapsa psammophilla Tamura, Iwataki et Horiguchi 2005 (
Reference: Tamura et al. 2005, pp 304–309, figs. 1–20.
Specimen examined: Slide LJB2012-08 at the NIBR, Incheon.
Description: Armored photosynthetic dinoflagellate with yellow-brown chloroplasts, spherical pyrenoid, and spherical nucleus situated in the hypotheca. Cells are ovoidal; almost equally sized epitheca (bell-shaped) and hypotheca (bowl shape). Width of the cingulum is approximately one-eighth to one-tenth the cell length and is displaced by almost half its width. Line drawing of this species is shown in Fig. 6A (Iwataki 2008).
Size: L (µm): 17–22 (21.5 ± 0.3); W (µm): 12–18 (17.8 ± 0.6).
>
Genus Amphidiniopsis Woloszy?ska 1929
Amphidiniopsis swedmarkii (Balech 1956) Dodge 1982 (
Basionym:
References: Dodge 1982, p 248, fig. 33D, 33E; Hoppenrath 2000b, p 483, figs. 4, 5, 19, 34–45.
Specimen examined: Slide LJB2011-17 at the NIBR, Incheon.
Description: Armored non photosynthetic dinoflagellate. Cells are rectangular, rounded posteriorly, dorsoventrally flattened. The cytoplasm contains colorless granules. Epitheca is cap-like and slightly narrow than the hypotheca. There are two large antapical spines. Two pusules lie in the lower right and upper left lateral part of the hypocone. The cingular and sulcal plates are smooth with scattered pores. Line drawing of this species is shown in Fig. 6E (Hoppenrath 2000b).
Size: L (µm): 48.5–52.8 (51.2 ± 2.4); W (µm): 38–44.5 (42.1 ± 1.8).
Amphidiniopsis rotundata Hoppenrath and Selina 2012 (
Reference: Hoppenrath et al. 2012, pp 155–167, figs. 1–21.
Specimen examined: Slide LJB2011-18 at the NIBR, Incheon.
Description: Armored nonphotosynthetic dinoflagellate. Cells rounded, dorsoventrally flattened. The smaller cap-like epitheca is slightly narrower than the hypotheca. The cingulum is slightly ascending about half of the cingular width. Sulcus distinctively curved and shifted to the left of the ventral side of the cell. One or two pusules are visible in living cells. Cells are colorless, and no food particles observed. Line drawing of this species is shown in Fig. 6I (Hoppenrath et al. 2012).
Size: L (µm): 22.5–36.8 (26.2 ± 1.4); W (µm): 21–34.5 (31.1 ± 0.8).
>
Genus Thecadinium Schiller 1933
Thecadinium kofoidii (Herdman) Schiller 1933 (
Synonyms:
Reference: Steidinger and Tangen 1997, p548, pl. 55A-B.
Specimen examined: Slide LJB2011-19 at the NIBR, Incheon.
Description: Armored photosynthetic dinoflagellate. Cells are oval and laterally compressed. Cingulum not displaced or may be slightly displaced. Nucleus is oval in the posterior part of the hypocone. Chloroplast appears to be single, radiating from the center of the cell.
Size of specimen: L (µm): 20–35 (24.6 ± 2.5); W (µm): 18–32 (22.8 ± 2.3).
Thecadinium yashimaense Yoshimatsu, Toriumi et Dodge 2004 (
Synonyms:
Reference: Yoshimatsu et al. 2004, p 216, figs 5, 31–38, 50.
Specimen examined: Slide LJB20110-20 at the NIBR, Incheon.
Description: Armored dinoflagellate with colorless cytoplasm, greenish food vacuoles. Cell dorsoventrally flattened, shape highly variable
Size: L (µm): 40–65 (51.6 ± 3.3); W (µm): 24–38 (30.8 ± 3.25).
>
Genus Cabra Murray and Patterson 2004
Cabra matta Murray and Patterson 2004 (
Reference: Murray and Patterson 2004, p 230, figs 1–18.
Specimen examined: Slide LJB2011-14 at the NIBR, Incheon.
Description: Armored non photosynthetic dinoflagellate. Cells are oval to rectangular and irregular and strongly laterally compressed. Flat hypotheca is much larger than the epitheca. Cell surface is areolate with many indentations and small pores. Cingulum is strongly ascending and incompletely encircles the cell. A large red body or food body is present. Line drawing of this species is shown in Fig. 6S (Murray and Patterson 2004).
Size of specimen: L (µm): 32–42 (34.5 ± 2.3); W (µm): 22–32 (28.8 ± 2.8).
Eighteen genera including 37 species of benthic dinoflagellates were identified during this study. The number of idenfied species is comparatively higher than previously published studies, such as Fukuyo (1981) (11 species), Turquet et al. (1998) (12 genera, 39 species), Parsons and Preskitt (2007) (26 species), Mohammad-Noor et al. (2007) (9 genera and 24 species), Selina and Levchenko (2011) (8 genera, 13 species, including five potentially toxic species), and Kim et al. (2011) (five genera, five species). This suggests that Jeju Island’s coastal waters contain diversified benthic dinoflagellate flora.
In our study, we identified 31 benthic dinoflagellates as new records for Jeju Island and Korean waters. These newly recorded species are previously unreported in the study area and have not been formally reported in scientific journals or monographs. Morphological observations of the benthic dinoflagellates from this study generally agreed with previous taxonomic descriptions from other geographical locations. The list of the species found in this study will add to the existing record on marine benthic dinoflagellates in Jeju coastal waters and can be used for future reference in phytoplankton studies.
The number of marine sand-dwelling dinoflagellates in the present study was less compared to studies by Carlson (1984) (46 species), Hoppenrath et al. (2003) (28 genera, 140 species), Murray (2003) (56 species), and Saburova et al. (2009) (43 taxa). However the total number from the present study was higher than that reported by Faust (1996, 2009). The differences in the number of sand-dwelling dinoflagellates could be attributed to several factors such as variation or uniqueness of the sampling station, period, or type of sediment. Sampling strategies (collection methods, seasonal distribution of species, sampling duration, etc) could also be responsible for the variation in the number of species identified (Hoppenrath and Elbrächter 1998). In addition, the presence of dinoflagellates is less in sediment compared to in the water column (Anderson and Kawachi 2005). It has also been reported that species richness in benthic and epiphytic dinoflagellate assemblages is usually lower than that of planktonic species (Okolodkov et al. 2007). Sand is a preferred habitat for benthic dinoflagellate species including surface macroalgae, algal turf, and detritus (Bagnis et al. 1985, Morton and Faust 1997, Aligizaki and Nikolaidis 2008). Fenchel (1988) reported that the inshore sand at South Water Cay provides an ideal habitat where benthic dinoflagellates divide among sand grains and proliferate in the nutrient-enriched benthic environment. It is also known that dinoflagellates seek shelter from meiofaunal predators (Faust 1996) in the interstitial spaces between sand grains. Faust (1990) reported the association between sand grains and biodetritus, benthic debris, and mangrove detritus. Furthermore, dinoflagellates are particle-associated forms that attach loosely to sand grains (Faust 1996). The presence of a number of sand dwelling benthic dinoflagellates suggests that the intertidal zones of Jeju Island coast are serving as potential nurseries or a seed-bank for dinoflagellates.
The presence of nine potentially toxic species, known to be causative of ciguatera fish poisoning
The existence of subtropical/tropical benthic dinoflagellates on Jeju Island is reflective of the changing climate from temperate to subtropical. We believe that the occurrence and distribution of tropical species highly reflects the flow pattern of the Tsushima Warm Current, which is affecting distribution more strongly than in the past, courtesy of the changing climate.
In conclusion, the results of this study have added to the existing dinoflagellate taxa including a large number of unrecorded and several potentially toxic species from sand sediment and macroalgae in the intertidal zone along the coasts of Jeju Island. Future studies should be carried out on the detailed taxonomy, molecular phylogeny, and seasonal abundance of benthic dinoflagellates to provide detailed information on the benthic dinoflagellates of Jeju Island.