Several kinds of alkaloids commonly occur in plants and have a wide range of pharmacological effects such as antioxidant and anticancer activities, pain relief, and promotion of blood circulation.1-3 There are various types of alkaloids in herbal medicines associated with diverse alkaloid metabolism in plants, but they are rarely identied. Therefore, there is a ongoing need for identication of biologically active compounds present inherbal medicines.
Structural characterization of alkaloids plays an important role in the drug discovery, understanding of metabolism and pharmacological effect as well as in quality control of herbal medicines. However, structural determination of the bio-active alkaloids is not an easy process due to many compounding factors (e.g., their structural diversity, the lack of authentic standards, and the complexity of herbal extracts). To date, numerous analytical methods have been developed, which may facilitate structural characterization of the alkaloids in herbal medicines, and include high-performance liquid chromatography (HPLC),4 LC/electrospray ionization (ESI) tandem mass spectrometry (MS/MS),5 capillary electrophoresis (CE)/ion spray mass spectrometry (MS),6 gas chromatography/mass spectrometry (GC/MS),7 and nuclear magnetic resonance (NMR).8
Among those analytical methods, LC/ESI-MS/MS equipped with a triple-quadrupole or ion-trap analyzer is the most popular technique for structural characterization of multiple components (such as alkaloids in complex herbal extracts) with high sensitivity and selectivity. In previous studies,9-12 the fragmentation behaviors of alkaloids have been intensively investigated using MS/MS to specify the structural patterns. To date, there have been limited studies on the specic alkaloids inherbal medicines.
Therefore, the present study adopted LC/ESI-MS/MS to identify various alkaloids observed in herbal medicines. The MS/MS spectra of several type alkaloids were investigated using the ESI-MS/MS technique to determine diagnostic ions depending on the structural characteristics of alkaloids in C. species. The spectral patterns enabled the characterization not only of all major alkaloids of interests, but also of minor and new compounds occurring in trace amounts. On the basis of the MS/MS fragmentation patterns of the structurally diverse alkaloids, a various types of alkaloids extracted from the herbal medicines could be identied and tentatively characterized by the LC/ESI-MS/MS technique. Moreover, the investigation of the fragmentation behavior of alkaloids yields a detail insight in the nature of fragment ions.
HPLC-grade methanol and acetonitrile were purchased from J.T. Baker (Phillipsburg, NJ, USA) and were used to prepare a standard stock solution and mobile phase. Authentic alkaloids included were either purchased from various chemical companies -Sigma Aldrich (Steinheim, Germany); Wako Chemicals (Osaka, Japan); and ChromaDex (Irvine, CA, USA) - or isolated by a previously reported method13-glaucine. All standards were kept in a refrigerator to keep them in a dry place.
All ESI-MS experiments were performed using an API 3200instrument (MDS Sciex, Concord, ON, Canada) equipped with an ESI source. All parameters were optimized according to the manufacturer’s instructions. In ESI-MS experiments, mass spectrometric conditions were as follows: curtain gas, 20 psi; electron voltage, 4500 V; temperature, 400℃ nebulizing gas, 50 psi; and heating gas, 50 psi; the mass scan range was m/z 100-400 in the full-mass scan. Collision energy was 40% in the Q2.Tandem mass spectra were obtained with low-energy collision-induced dissociation (CID). A syringe pump was used for direct analysis of the nine reference compounds at a flow rate of 10 mL/min. The [M + H]+ or [M]+ ions were selected as precursor ions in the positive-ion ESI mode.
>
ESI-MS/MS of authentic alkaloids
For the structural determination of various types of alkaloids extracted from herbal medicines, eight type alkaloids were analyzed by ESI-MS/MS to find the specificity of MS/MS spectral patterns according to their structural characteristics. These heterocyclic alkaloids with secondary or tertiary amine groups are composed of four rigid rings with different skeletons. The ESI mass spectra of most alkaloids showed abundant [M+H]+ ions due to the strong basicity of the secondary amine group. On the other hand, protoberberine alkaloids with the cation on the nitrogen atom produced intact [M]+ ions with strong intensities in the ESI-MS analysis. Thus, these ions were selected as precursor ions for collision-induced dissociation (CID) experiments in positive-ion mode.
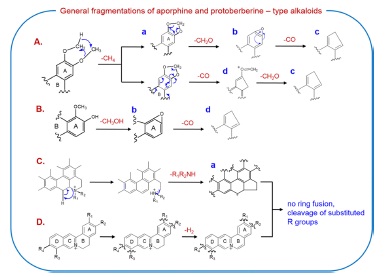
Most of these alkaloids possess methylenedioxyl or methoxylgroups at the C2-C3 and C9-C10 positions, respectively. All isoquinoline alkaloids with vicinal methoxyl groups at the A- and/or D-ring produced some common product ions: [M+H − CH4]+ (a-type), [M+H − CH4 − CH2O]+ (b-type), [M + H − CH4 − CO − CH2O]+ (c-type), and [M+ H − CH4 − CO]+ (d-type) ions (Scheme 1-A). In addition, isoquinoline alkaloids with vicinal methoxyl and hydroxyl groups also produced the characteristic ion formed by the loss of a CH3OH molecule, as shown in Scheme 1-B.
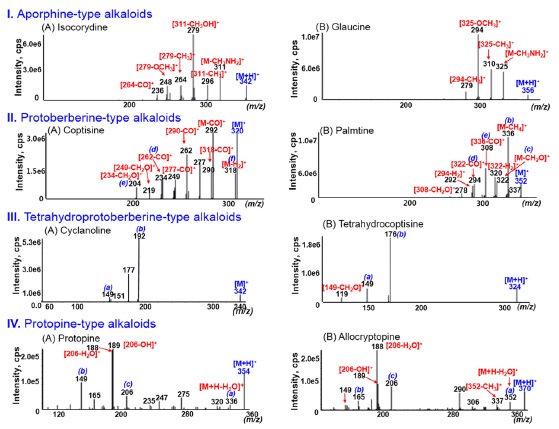
The CID-MS/MS spectra of aporphine alkaloids can be characterized by the presence of a-type ion and the cleavage of substituted groups (Scheme 1-C). The initial fragmentation was the loss of methylamine to form a five-membered cyclic ring, which was already reported by previous studies.14 After demethyamination, no product ions by further ring fusion were observed and the product ions were mainly formed by the successive dissociation of substituted groups on rigid ring. The dissociation of substituted groups follows the same pathways shown in Scheme 1-A and B. Thus, the MS/MS spectra of [M + H]+ ions for isocroydine and glaucine (Fig.1-I) displayed some product ions due to cleavage of substituted groups, but no
characteristic ions below m/z 220.
For the MS/MS spectra of protoberberine alkaloids containing a rigid C-ring structure, no significant ions produced by ring fusion were observed, and the characteristic ions were formed mainly by the cleavage of substituted groups and dehydrogenation (Scheme 1-D). The MS/MS spectra of [M]+ ions for coptisine and palmatine (Fig.1-II), respectively, showed no fragment ions observed below m/z 200. These characteristic ions of protoberberine alkaloids were also formed above m/z 200 by the successive cleavages of substituted methoxyl or methylenedioxyl groups on the A-and D-rings. Interestingly, the characteristic ions were detected as dehydrogenated ions such as [M − H2]+ (m/z 318 for coptisine) and [fragment ion −H2]+ ions (m/z 290 for coptisine and m/z 320 and 292 for palmatine), which form a stable conjugated structure at the B-ring. Observation of dehydrogenated product ions may help differentiate protoberberine-type alkaloids from other types of alkaloids.
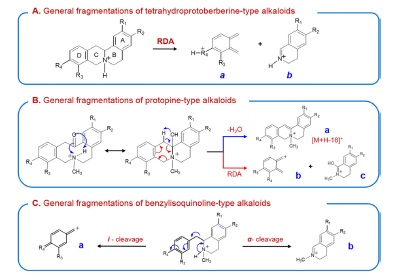
The MS/MS spectra of tetrahydroprotoberberine alkaloids showed wide spectrum range, as shown in Fig. 1-III. The formation of product ions could be explained in two ways. The product ions above m/z 280 were mainly formed by cleavage of the substituted groups on the alkaloid ring. The characteristic ions below m/z 200 were mainly produced by both retro-Diels-Alder (RDA) fragmentations. The diagnostic RDA fragmentations (a- and b-type ions in Scheme 2-A) enabled structural determination of the A- and D-ring substitution patterns as well as the isoquinoline moiety and complementary moiety. Moreover, these fragmentation patterns could facilitate discrimination of other types of alkaloids in MS/MS analysis. The RDA fragmentation for tetrahydroprotoberberine alkaloids was also already reported by other researchers.15
Several common MS/MS product ions of protopine-type alkaloids were produced by various dissociation processes such as RDA fragmentation, dehydration through iminolization,
and successive dissociation of substitute groups, as shown in Scheme 2-B. Especially, the observation of the a-type [M+H −H2O]+ ion suggests that the protopine-type alkaloids can be converted into tetrahydroprotoberberine alkaloids through the iminolization process during the ESI process and then lose the water molecule in the CID process. A plausible fragmentation mechanism for the dehydration of protopine alkaloids has been suggested in our previous literature.16 The characteristic b- and c-type ions were produced through iminolization and RDA fragmentation. The MS/MS spectra of protopine and allocryptopine as protopinetype alkaloids are shown in Fig. 1-IV.
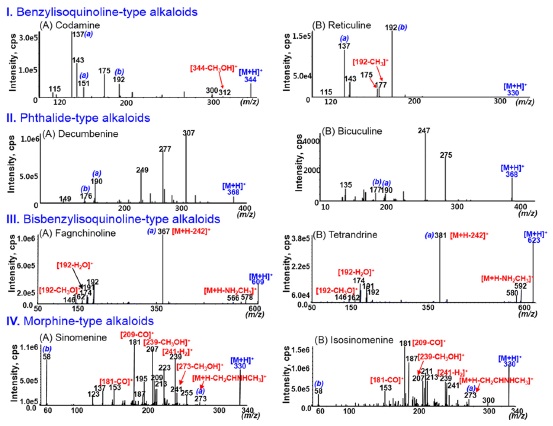
The MS/MS-spectra of the benzylisoquinoline-type alkaloids showed a very weak intensity of the [M+H]+ion due probably to their less rigid structure compared to other-type alkaloids (Fig. 2-I). Especially, major characteristic ions were formed by inductive cleavage and á-cleavage at the nitrogen of the tyramine moiety, as indicated in Scheme 2-C. The formation of these ions for benzylisoquinoline alkaloids were already reported in previous literature.17
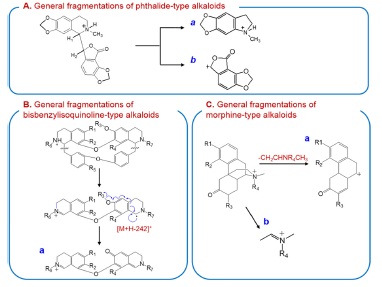
The MS/MS fragmentation pathways of the [M+H]+ ion for phthalide alkaloids are suggested in Scheme 3-A. The MS/MS spectra of the phthalide-type alkaloids showed various characteristic ions due to the successive losses of CH3NH2,CH2O, and/or H2 molecules, and the bond cleavage between isoquinone (a-type) and phthalide ring [b-type]. The MS/MS spectra of decumbenine and bicuculine as phthalidetype alkaloids are shown in Fig. 2-II. The formation of abundant ions at m/z 307, 277, 275, 247, and 249 were explained in detail in our previous literature.16
The MS/MS spectra of [M + H]+ ions for bisbenzylisoquinoline alkaloidscould be characterized by the presence of the most abundant [M + H −242]+ ions and several fragment ions below m/z 192, as shown in Fig. 2-III. The [M + H − 242]+ ions were produced by the preferred cleavage of the
carbon-carbon bond both β to nitrogen and β to the two aromatic systems followed by the O-demethylation of an aromatic methoxy group (a-type ions in Scheme 3-B).18,19 The ions at m/z 192 could provide the structural information of isoquinoline moiety.
The MS/MS spectra of morphine alkaloids with the 4,5epoxy bridge showed wide range of fragment ions by various cleavages (Fig. 2-IV). The characteristic ion [M + H − C3H6]+ (a-type in Scheme 3-B) was formed by the loss of 4,5-epoxy bridge from protonated molecule [19]. The MS/MS spectra of morphine-type alkaloids can be characterized by the presence of complex product ions above m/z 120, except for ion at m/z 58 (b-type). The other fragment ions might be formed by the successive cleavage of substituted groups on rigid ring.
In this study, MS/MS spectral patterns of aporphine-, protopine-, benzylisoquinoline-, bisbenzylisoquinoline, phthalide-, tetrahydroprotoberberine-, morphine-, and protoberberine- type alkaloids were characterized by low-energy CID dissociation. Structurally diagnostic ions were suggested for various types of alkaloids observed in herbal medicines. These ESI-MS/MS spectral patterns can be useful for the determination of known and unknown alkaloids in herbal medicines. This study may also facilitate understanding of biosynthesis and metabolism of alkaloids in herbal medicines as well as pharmacological effect.