Nitrogen is a one of the most vital nutrients for aquatic plants and algae. However, an excessive concentration of nutrients (containing nitrogen) will stimulate aquatic plant and algal growth and will cause serious pollution problems. In order to prevent these problems, we should handle the nitrogen as a nutrient resource rather than a pollutant that only has to be disposed off. Biological treatment using microalgae is one of the potential treatments to reduce the nitrogen, where the nitrogen is used as a nutrient for the microorganisms.
Many algal species, especially the family of
One limitation in employing an algal system as the secondary treatment process is the presence of high concentrations of ammonia and urea in raw wastes, especially those discharged from the livestock and food industries, which inhibit algae growth and physiological activity [7]. However, it has been noted that the studies undertaken previously were mainly focused on the effects of N-deficiency, and competitive interaction between nitrate and ammonia uptake at low N level. Relatively little information is available on ammonia removal using different ammonia concentrations in real wastewater treatment by
Based on the aforementioned reasons, in the present investigation we have aimed to explore and examine the efficiency of
2.1. Microalgae Cultures, Medium and Chemicals
Cells of
2.2. Characterization of Wastewater
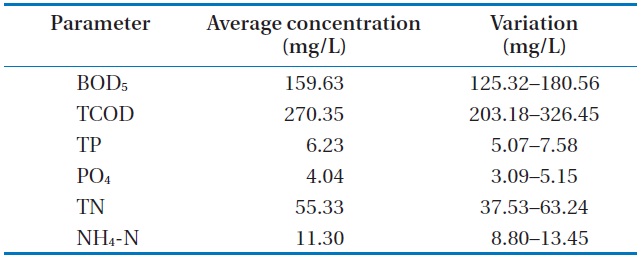
The raw wastewater was obtained from the preliminary sedimentation of a sewage plant at Gangneung, Korea. Table 1 shows the characteristics of the wastewater used throughout our investigation.
The analyzed raw wastewater was noted to be favorable for treatment with microalgae and removal of the available nutrients. An excess ratio of chemical oxygen demand, nitrogen, and phosphorus (i.e., 100:20:2) for this wastewater is recommended for nutrient removal in activated sludge plants. The biochemical oxygen demand (BOD5) and total phosphorus (TP) and BOD5 and PO4 ratios were found to be reasonably high. Similarly, the PO4 and TP ratio was at its higher range for municipal wastewater. Lastly, calcium, potassium and manganese were not limiting for biological wastewater treatment, and iron was naturally present in the wastewater.
2.3. Experimental Design and Batch Cultivation Method
To eliminate bacteria and protozoa, the wastewater samples were sterilized by autoclaving for 30 min. The experiments were conducted using a batch reactor operation with 1 L conical flasks. At the beginning of each series of experiments, 500 mL of wastewater was inoculated to the flasks with pre-cultured
The initial cell density was 1 × 106 cells per milliliter for each experimental set up. The initial chlorophyll
The pH values of the cultures were measured intermittently and maintained a constant value of pH (7.5 ± 0.3) by the addition of sterilized and diluted NaOH or HCl.
Dry-weight estimations do not exclusively monitor the amount of algae, because bacteria and zooplankton may add to the biomass. Because only algae contain chlorophyll, the estimation of this pigment was a reliable though elaborate method in algae biomass computation. Depending on the algal strain examined, acetone, ethanol, or diethyl ether was used to extract the pigment from the separated algal cells. In some cases, brief heating was required to achieve complete pigment extraction. After that, the cell debris was removed by centrifugation or filtration, and the extract was protected from light to avoid bleaching of the pigments.
The Chl
The experiment was conducted for a maximum of 8 days contact. The specific rate of NH4-N removal (Rs) was also estimated, using the known Eq. (2):
where (Chl
NH4-N concentration was measured by ion chromatograph (Metrohm AG, Herisau Switzerland), and the analysis was performed according to the standard methods described elsewhere [9].
[Table 1.] Characteristics of the raw wastewater
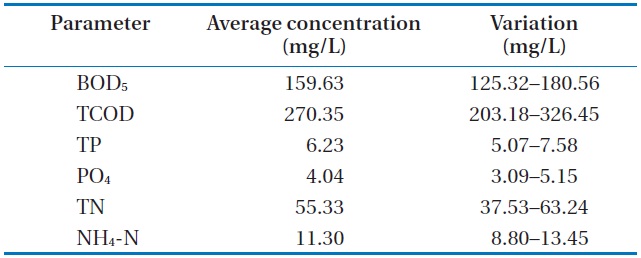
Characteristics of the raw wastewater
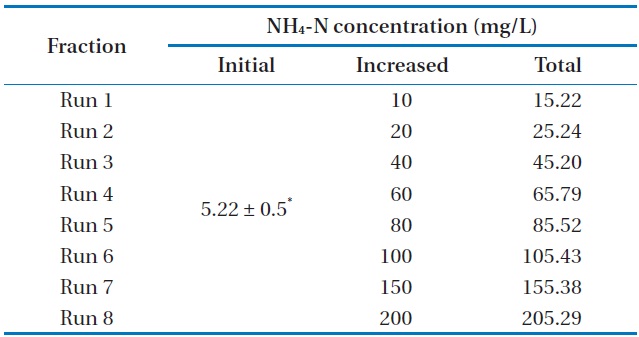
[Table 2.] Characteristics of the experimental design
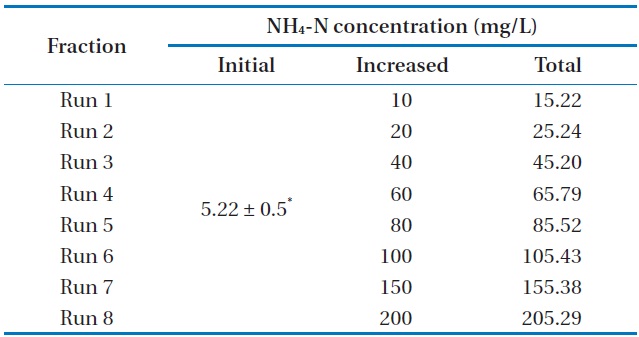
Characteristics of the experimental design
3.1. Efficiency of NH4-N Removal
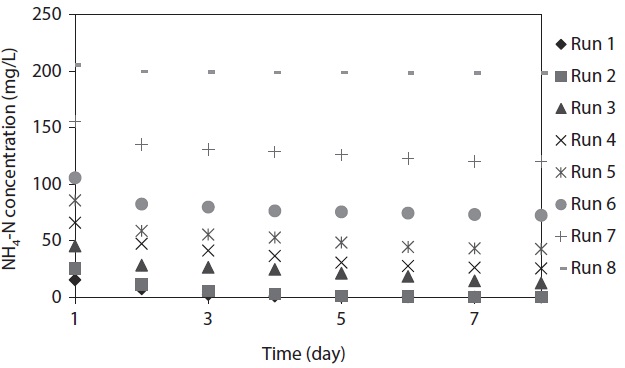
The variation of NH4-N concentration as a function of time with various initial NH4-N concentrations is depicted in Fig. 1.
The maximum NH4-N removal efficiency was obtained after 8 days, and the values were found to be 99.61%, 99.52%, 72.32%, 61.32%, 50.22%, 31.31%, 22.99%, and 3.59% for Runs 1–8, respectively. NH4-N was completely removed by
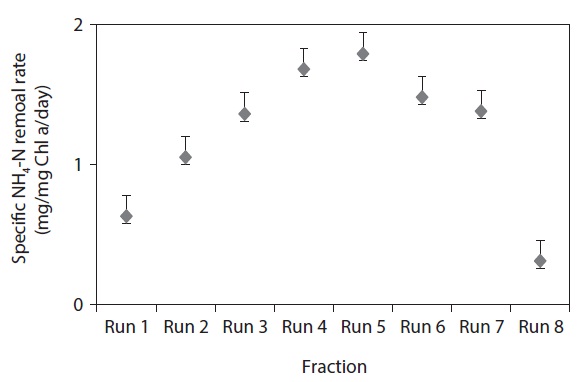
3.2. Chl a and Specific NH4-N Removal Rate
Nitrogen is the major constituent of proteins, chlorophyll, and enzymes involved in photosynthesis. Therefore, nitrogen affects the photosynthesis of microalgae. The nitrogen absorbed by
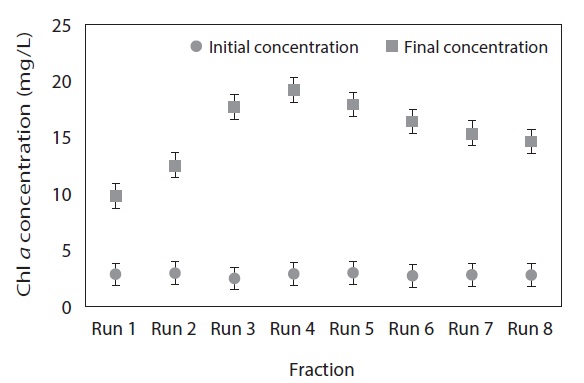
Fig. 2 clearly demonstrates that the final Chl
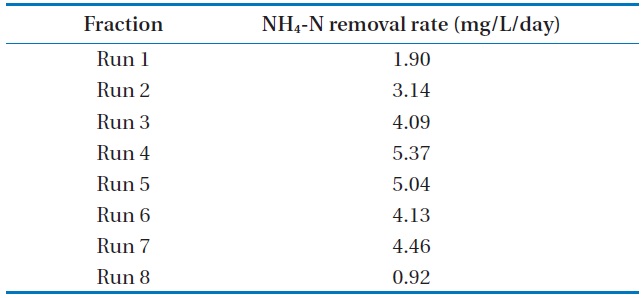
The batch data was further utilized to discuss the kinetics of NH4-N removal. The initial NH4-N removal rate was used to determine the coefficients. The removal rate (R) was calculated for these wastewater samples, and the obtained values are represented in Table 3.
The maximum NH4-N removal rate was found to be 5.37 mg/L/day for Run 4. Run 4 sample was obtained 2.8 times and 5.8 times higher than that of Run 1 and Run 8, respectively. The
NH4-N removal rates were similar to those obtained previously by other related studies. The nitrogen removal rate by
The initial cell density was 1 × 106 cells per milliliter, and the initial Chl
NH4-N absorbed by
[Table 3.] NH4-N removal rate at various NH4-N concentrations using Chlorella vulgaris
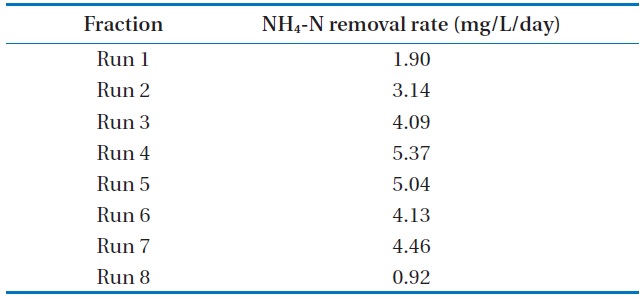
NH4-N removal rate at various NH4-N concentrations using Chlorella vulgaris
dissipate the excessive energy. So they cannot efficiently utilize the photon energy absorbed by pigments for photosynthesis. NH4-N partly replacing NO3-N decreases the consumption of energy and reducing power, while NO3-N partly replacing NH4-N relieves metabolic disorder induced by the excessive NH4-N, and makes the physiological metabolism in
In this study, the potential of