Polymer composites with both electrical and ferromagnetic properties have attracted significant consideration. The conducting polymer composites are attractive, due to their multifunctional applications in batteries, electro-chemical display devices, electric magnetic shielding material, microwave-absorbing materials, etc. [1-3]. Polypyrrole (PPy) is one of the conducting polymers, because of its high conductivity, electrical properties, and ease of production [4,5]. It is well known that PPy matrix can effectively reflect electro-magnetic waves generated from an electric source, and the electro-magnetic waves from a magnetic source can be shielded by using magnetic materials [6,7]. Thus, the reinforcement of magnetic fillers into PPy matrix can generate multifunctional composites, with new physical properties [8]. The research on microwave-absorbing materials continues to attract much attention, and ferrites is a popular material. But the use of ferrite-based absorbers requires higher performance at higher frequencies, such as X-band [9,10]. Among the conventional microwave absorption materials, the spinel-type ferrites are popular. However, spinel-type ferrites show Snoek’s limit, and the magnetic loss decreases at giga-hertz range [11-14]. Earlier, spinel ferrites have been most frequently utilized as absorbing materials, in various forms. Manganese ferrite (MnFe2O4) is a common spinel ferrite material, and has been widely used in microwave and magnetic recording applications. However, it has been shown that magnetic composites are useful as microwaveabsorbing materials, due to their advantages in respect of lightweight, low cost, design flexibility, and microwave properties. In the present work, MnFe2O4 is reinforced in the PPy matrix, and its effect on electrical and ferromagnetic properties is compared with the pristine form.
The chemicals, including metal salts, hexamethylenetetramine (HMTA), potassium persulfate (KPS) and ethylene glycol (EG), were analytical grade from Sigma, and were used without further purification. Water was deionized, doubly distilled, and deoxygenated, prior to use. The styrene and methacrylic acid were purchased analytical grade from Sigma, and were distilled to remove the inhibitor. The pyrrole monomer was distilled twice, under reduced pressure. The DBSA and acrylic resin were of industrial grade. The other reagent, including iron chloride (FeCl3), was of analytical grade Sigma.
Negatively charged PS spheres with average diameter 230 nm, which were used as core particles, were prepared by a freeemulsion polymerization method. In a typical experiment, 10 ml styrene, 2 ml methacrylic acid, and 0.055 g KPS were added to a flask with 100 ml de-ionized water. To eliminate oxygen effects, the solution was purged with nitrogen, before the process was initiated. The mixture was heated to 75℃, and stirred with a magnetic stirrer. The polymerization was continued for 24 h, and in the whole procedure, the nitrogen was purged. The concentration of PS spheres in solution was 80 mg/ml, which was calculated by drying 4 ml colloid solution, and weighing the remained solids.
The coating technique consisted of controlled hydrolysis of aqueous solutions of ferrous chloride and other divalent metal salts, in the presence of polystyrene latexes. In a typical preparation process, 2 ml PS colloid solution was diluted with 300 ml deoxygenated distilled water, and then mixed with the metal salts solution, which contained 10 m.mol FeCl2 and 5 m.mol MnCl2. After dispersal under ultra-sonic for several minutes, the mixture was incorporated with 4 g HMTA and 0.5 g potassium nitrate, and heated to 90℃, under gentle stirring. After 3 h, the system was cooled to room temperature. The solution was poured into excess distilled water, then magnetic particles were deposited, using a magnetic field. The precipitate was washed with distilled water several times, and then dried in oven, at 75℃ for 24 h. In addition, to modify the surface chemical properties of the composites magnetic spheres, 5 ml ethylene glycol (EG) was added into the reaction solution, before the incorporation of HMTA.
The MnFe2O4-PPy core-shell composites were prepared by
The morphology of coated particles and composite was observed by transmission electron microscopy (TEM), with a Hitachi instrument operated at an accelerating voltage of 10 kV. The X-ray powder diffraction (XRD) patterns of the nanoparticles assemblies were collected on a Philips-PW 1800 with Cu-K radiation, under CuKα radiation (λ = 1.5406 A). The fourier transform infrared spectroscopy (FTIR) spectra were recorded on a PerkinElmer spectrum FTIR, using KBr pellets. The M-H hysteresis loops were measured by vibrating sample magnetometer (VSM) (Riken Denshi Co. Ltd., Japan). Electromagnetic radiation reflectivity measurements, over the frequency range of 8~12 GHz, were done using a waveguide, coupled to an Agilent Synthesized Sweeper model 8,375 A, and a HP 7000 spectrum analyzer.
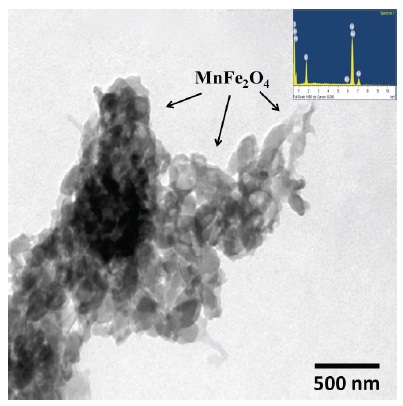
A TEM micrograph of the 10 wt% MnFe2O4 reinforced PPy composite is shown in Fig. 1. The microstructure of the composite shows the complex networking of MnFe2O4 fillers in the PPy matrix. The inset of Fig. 1 shows EDS composition of the MnFe2O4 particles, indicating the high-quality dispersion of the fillers within the PPy matrix.
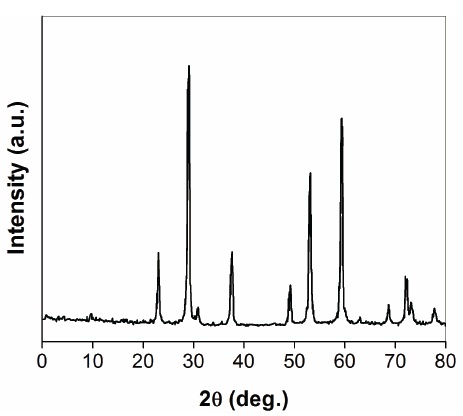
Figure 2 shows the XRD spectrum of manganese ferrites nanofillers. It can be clearly observed from the XRD spectrum that the ferrite shells are phase-pure spinel structure in all cases, according to the standard XRD spectra of spinel ferrite Fe3O4 (2 θ = 35.5, 43.7, 57.0, 62.4, 74.0, 78.0), Mn ferrite (2 θ = 33.0, 71.0, 86.4), and NaCl (2 θ= 75.5). The average crystallite size was calculated using JCPDS, through the diffraction peaks from Scherer’s equation [15].
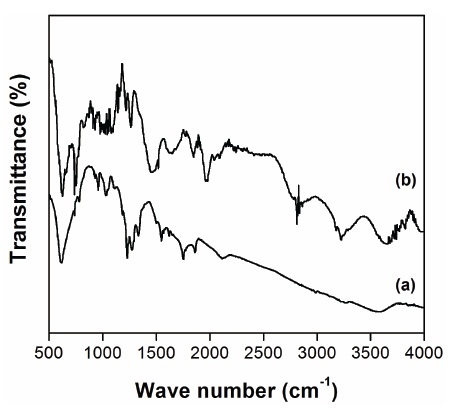
In Fig. 3 show the FT-IR spectra of MnFe2O4 and PPy-MnFe2O4 composite. It was observed from Fig. 3 that the peak at 571 cm-1 is the intrinsic vibrations of manganese ferrite. The characteristic peaks of styrene occur at 811, 865, 1,009 to 1,382, 1,458 and 1,638 cm-1. The peaks at 811 and 865 cm-1 are related to the C-H outer bending vibrations. The peak at 1458 cm-1 is attributed to the characteristic C=C stretching ring. The peak at 1,638 cm-1 is attributed to the styrene ring.
As shown in (b) of Fig 3, the characteristic peaks of PPymanganese ferrite composite occur at 576, 670, 908, 959, 1,006, 1,038, 1,188, 1,313, 1,461, 1,555 and 2,920 cm-1. However, the characteristic peaks of MnFe2O4 for F-O and Mn-O stretching vibrations can be observed at wave numbers 576 and 670 cm-1. The peaks at 908 to 1,006 cm-1 are attributed to the p-disubstituted aromatic ring C-H out-of-plane bending. The peak around 1,038 cm-1 is associated with vibrational modes of N=Q=N (Q refers to the quinonic type rings), indicating that PPy is formed in our sample. The peaks at 1,461 and 1,555 cm-1 are attributed to the characteristic C=C and C-N stretching of polypyrrole ring; the peaks at 1,188 and 1,313 cm-1 correspond to N-H bending and asymmetric C-N stretching modes of the PPy ring.
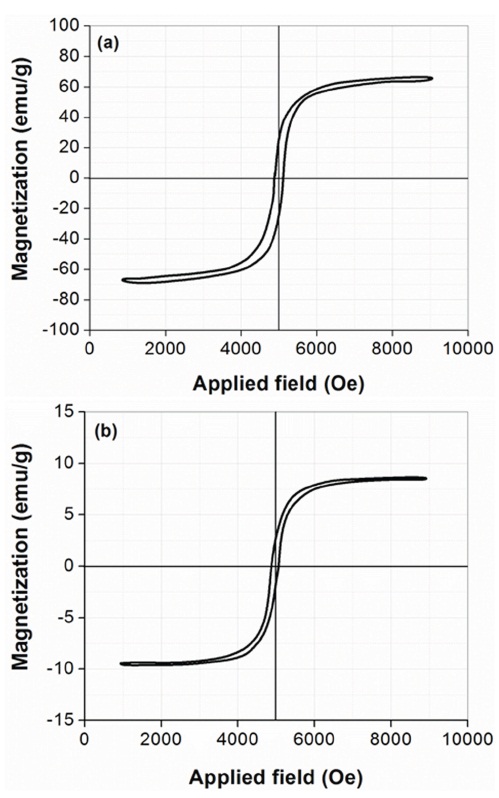
Figures 4 (a)~(b) show the magnetization (M) versus the applied magnetic field (H) for MnFe2O4 and MnFe2O4/PPy composite (10 wt%), respectively. The magnetic properties of the ferrite-coated PS latex were analyzed by room temperature VSM, with an applied field of -10≤
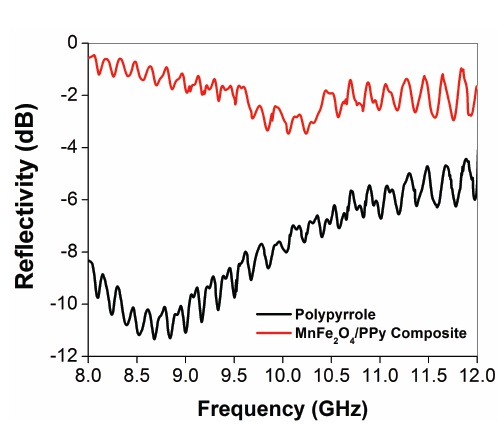
In Fig. 5, the results of reflectivity (in decibels, dB) are presented as a function of frequency (GHz). In the present study, the permittivity data are not available for the samples; thus, comparison with theoretical reflection curves is not possible. High radiation attenuation values (ca. -9.5 dB), in the frequency range of 11~12 GHz, were obtained in the PPy matrix. Also, the composite containing 10 wt% of composite presented radiation attenuation values between -1 and -3 dB, in the frequency range of 8~12 GHz. In this case, the composite exhibits a broadband behavior, with microwave radiation absorption of 80%. We can observe that the incorporation of MnFe2O4 as reinforcement in the PPy matrix leads to a shift of the attenuation values to lower frequencies, and the composite attained high radiation attenuation values
(ca.-10 dB), in the frequency range of 8~10 GHz, corresponding to an attenuation between 75 and 95% of the incident radiation. This is due to the dielectric characteristics of polypyrrole. In other words, minimal reflection of the microwave power or matching conditions occurs. This shows that by reinforcement of MnFe2O4 nano-fillers, the attenuation peak frequency of composites can be manipulated.
The MnFe2O4 magnetic nano-fillers are of size 25.43 nm. PPymanganese ferrite composite with magnetic behavior is successfully synthesized by