Red tides―discoloration of the surface of the sea due to the blooms of plankton―constitute one of the most important environmental issues globally. By altering the balance of food webs and causing large-scale mortalities of fish and shellfish, red tides often lead to considerable losses in the aquaculture and tourist industries (Whyte et al. 2001, Curtiss et al. 2008, Richlen et al. 2010, Jeong and Kang 2013, Park et al. 2013c). Thus, many countries are endeavoring to understand the process of red tides, and thereby predict and control their outbreaks (e.g., Mackey et al. 1996). Red tide dynamics are known to be influenced by diverse physical, chemical, and biological properties (Cole 1982, Doucette et al. 1998, Imai et al. 2001, Han et al. 2010, Tang and Gobler 2010, Jeong et al. 2013a, 2013b, Kang et al. 2013, Kim et al. 2013a, 2013b, Lee et al. 2013, Park et al. 2013b, Yih et al. 2013, Yoo et al. 2013).
Several investigations have revealed that certain bacteria kill red tide organisms, thereby playing an important role in the decline of red tides (Imai et al. 1993, 2001, Doucette et al. 1998, Salomon and Imai 2006). Thus, the killing of red tide organisms by algicidal bacteria has been extensively studied (Fukami et al. 1992, Mayali and Doucette 2002). Meanwhile, in the last 2 decades, many red tide organisms, including phototrophic dinoflagellates and raphidophytes, have been shown to feed on bacteria (Nygaard and Tobiesen 1993, Seong et al. 2006, Jeong et al. 2010a, 2010b, 2010c, Jeong 2011, Park et al. 2013a). The predator-prey relationships of red tide organisms and bacteria (2 major components of marine environments) are therefore reversible.
In Korea, red tides have led to considerable losses in the aquaculture industries (Park et al. 2013c). Thus, methods to control the outbreak and persistence of red tides, and thereby reduce their economic impacts, are urgently required. Several potential control methods have been suggested or implemented (Jeong et al. 2002, 2003a, 2008, Sengco and Anderson 2004, Park et al. 2013c), including the use of mass-cultured algicidal bacteria.
Here, we review the species and concentrations of algicidal bacteria that kill red tide organisms in Korean waters, as well as the ingestion rates and grazing impact of red tide organisms on bacteria. Furthermore, we examine the ecological significance of the interactions between these 2 components of marine environments.
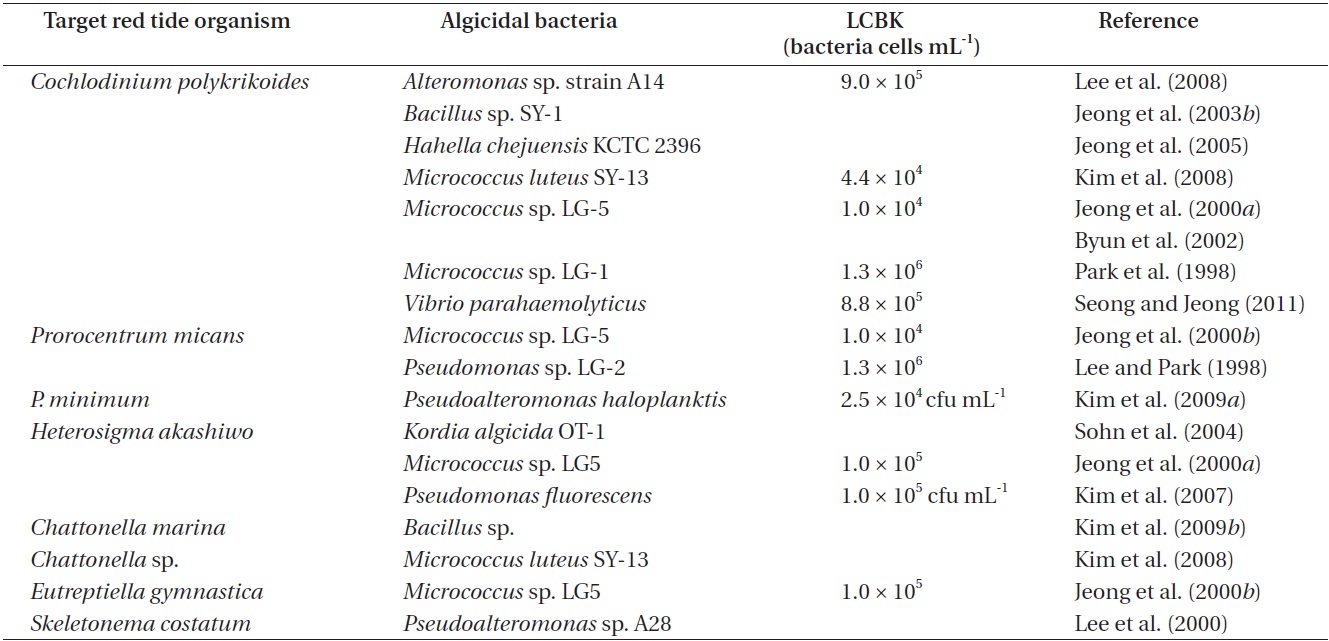
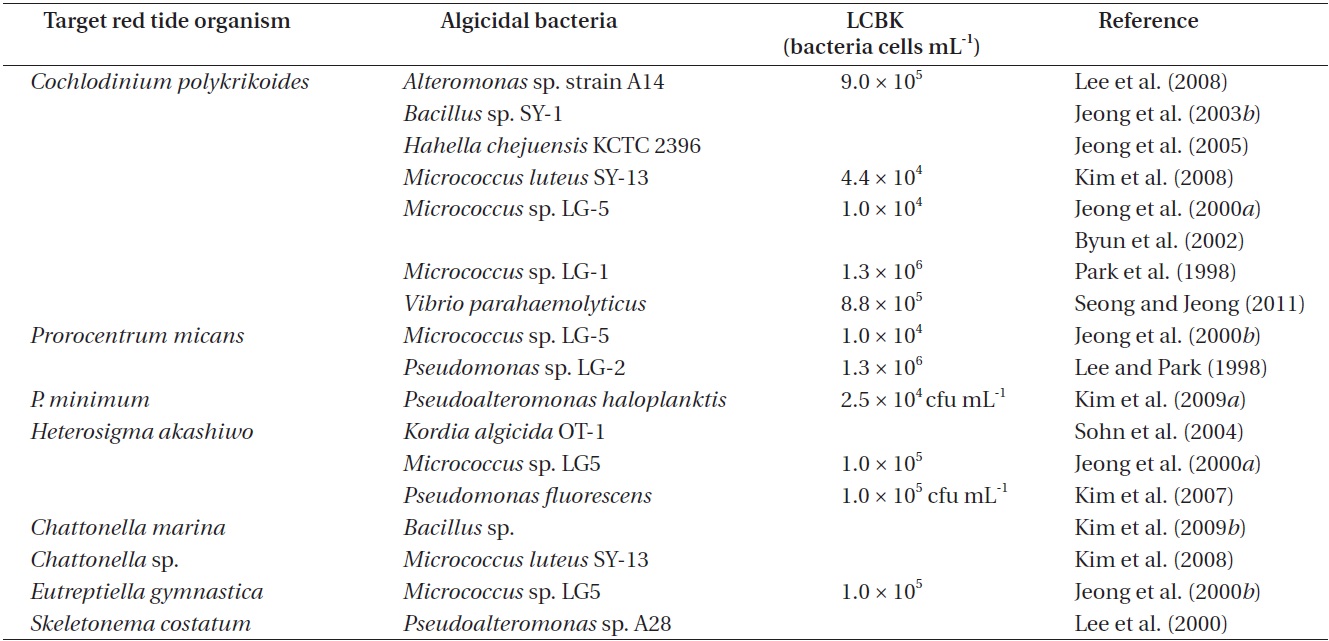
Many bacteria are known to kill red tide organisms in Korean waters (Table 1). In particular, algicidal bacteria that kill the mixotrophic dinoflagellate Cochlodinium polykrikoides, which causes considerable great losses in the Korean aquaculture industry, have been extensively studied (Jeong et al. 2004, 2008, Park et al. 2013c). Such
bacteria include Alteromonas sp. strain A14, Alteromonas sp., Bacillus sp. SY-1, Hahella chejuensis KCTC 2396, Micrococcus sp. LG-5, Micrococcus luteus SY-13, Nautella sp., Pseudomonas sp. LG-2, Pseudoalteromonas sp., Sagittula sp., Thalassobius sp., and Vibrio parahaemolyticus (Table 1). The algicidal components of these bacteria were revealed to be Bacillamide and Prodigiosin (Jeong et al. 2003b, 2005). In addition, algicidal activity usually peaked at 15 hours and was subsequently maintained (Park et al. 1998, 1999, Jeong et al. 2003b, Oh et al. 2011).
The bacterium Pseudoalteromonas haloplanktis was shown to lyse the cell wall of another mixotrophic dinoflagellate, Prorocentrum minimum; the algicidal activity was revealed to be caused by the release of β-glucosidase (Kim et al. 2009a). Furthermore, Pseudomonas sp. LG-2 was shown to kill the mixotrophic dinoflagellate Prorocentrum micans (Table 1). In addition, Pseudomonas fluorescens and Kordia algicida OT-1 are known to kill the raphidophyte, Heterosigma akashiwo, while Bacillus sp. is known to kill another raphidophyte, Chattonella marina. Bacillus sp. was also shown to kill the mixotrophic dinoflagellates Akashiwo sanguinea and Scrippsiella trochoidea, and the raphidophytes Fibrocapsa japonica and H. akashiwo (Table 1).
Pseudomonas sp. LG-2 killed Prorocentrum micans, but did not kill Alexandrium tamarense, Akashiwo sanguinea, Cochlodinium polykrikoides (Lee and Park 1998). In addition, Pseudoalteromonas haloplanktis killed Prorocentrum minimum and P. donghaiense, but did not kill A. sanguinea, A. tamarense, C. polykrikoides, Gymnodinium catenatum, and Heterosigma akashiwo (Kim et al. 2009a). However, Micrococcus sp. LG-5 killed diverse algae such as C. polykrikoides, H. akashiwo, P. micans, and the euglenophyte Eutreptiella gymnastica (Jeong et al. 2000a, 2000b). Thus, some algicidal bacteria kill specific species of red tide organisms, while others kill a diverse range of red tide organisms.
On the basis the results of laboratory and field experiments, methods to control red tides using mass-cultured algicidal bacteria have been developed (e.g., Kim et al. 2009a). The efficiency of these methods requires the determination of the minimum concentrations of algicidal bacteria. In addition, the efficacy in mesocosms and natural environments must be evaluated.
The lowest concentrations of algicidal bacteria required to kill target red tide organisms (LCBK) differ depending on the species of bacteria and red tide organisms (Table 1). For example, the LCBK of Micrococcus luteus SY-13 on C. polykrikoides was 3.7 × 103 cells mL-1, whereas those of Vibrio parahaemolyticus, Micrococcus sp. LG-1, and Alteromonas sp. A14 ranged from 0.9 × 106 to 1.3 × 106 cells mL-1 (Table 1). In addition, the LCBKs of Pseudomonas sp. LG-2 and Micrococcus sp. LG-5 on P. micans were 1.3 × 106 and 1.0 × 106 cells mL-1, respectively. Thus, before
using the control methods in natural environments, the LCBK must be determined.
The use of algicidal bacteria to kill red tide organisms has frequently been investigated by means of field mesocosms (Kim et al. 2009a). For example, in mesocosms containing P. minimum red tide water, P. haloplanktis AFMB-08041 was found to reduce the concentration of P. minimum from 1.5 × 104 to 2.3 × 103 cells mL-1 over 5 days (Kim et al. 2009a). Similarly, the algicidal bacterium Micrococcus sp. LG-1 (when applied at a concentration of 104 to 105 cells mL-1) reduced the concentration of C. polykrikoides from 4.8 × 103 to 2.0 × 102 cells mL-1 in Masan Bay (Park et al. 1998). However, before applying the method in large-scale field studies, it is important to investigate possible secondary effects on non-target organisms.
Mixotrophic red tide dinoflagellates. Many mixotrophic red tide dinoflagellates isolated from Korean waters are known to feed on bacteria (Table 2). For example, Amphidinium carterae, Alexandrium catenella, A. tamarense,
[Fig. 1.] Ingestion rates (IR; cells alga-1 h-1) of red tide dinoflagellate (A) and raphidophyte (B) on bacteria as a function of the initial prey concentration. IR values and regression curves were obtained from Seong et al. (2006). Pm, Prorocentrum minimum; Cp, Cochlodinium polykrikoides; Hr, Heterocapsa rotundata; Ht, Heterocapsa triquetra; Co, Chattonella ovate; Ha, Heterosigma akashiwo. Equations: IR = 21.9 [x/(23.9 × 106+ x)], r2 = 0.668 for Pm; IR = 17.4 [x/(26.3 × 106 + x)], r2 = 0.864 for Cp; IR = 6.0 [x/(3.2 × 106 + x)], r2 = 0.743 for Ht; IR = 11.2 [x/(9.4 × 106 + x)], r2 = 0.709 for Hr; IR = 24.5 [x/(7.2 × 106 + x)], r2 = 0.703 for Co; IR = 11.7 [x/(4.3 × 106 + x)], r2 = 0.771 for Ha.
Akashiwo sanguinea, Cochlodinium polykrikoides, Gonyaulax polygramma, Gymnodinium aureolum, G. catenatum, G. impudicum, Heterocapsa rotundata, H. triquetra, Lingulodinium polyedrum, Prorocentrum donghaiense, P. micans, P. minimum, P. triestinum, and Scrippsiella trochoidea are able to feed on heterotrophic bacteria (Seong et al. 2006, Jeong et al. 2010b). Recently, the zooxanthellae Symbiodinium spp., isolated from Korean waters, were also shown to feed on heterotrophic and autotrophic bacteria (Jeong et al. 2012). Thus, feeding of mixotrophic dinoflagellates on bacteria is common, regardless of genera, size, shape, presence of theca, etc. However, feeding by other mixotrophic red tide dinoflagellates on heterotrophic bacteria has not yet been investigated.
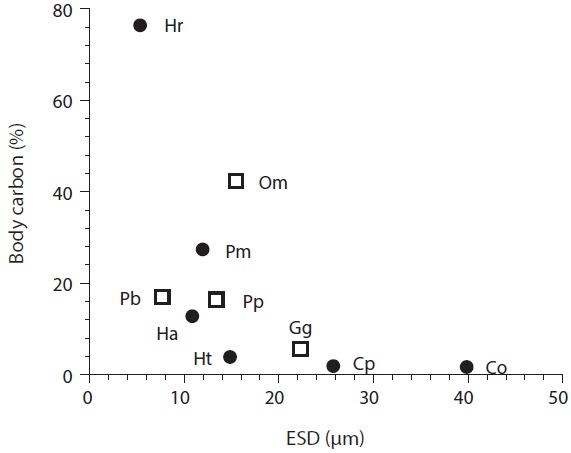
[Fig. 2.] Amount of carbon acquired by mixotrophic (circles) and heterotrophic (squares) dinoflagellates as a function of algal predator size. Body carbon (%), percent of acquired carbon from bacteria to predator’s body carbon; ESD, equivalent spherical diameter; Pm, Prorocentrum minimum; Cp, Cochlodinium polykrikoides; Ht, Heterocapsa triquetra; Hr, Heterocapsa rotundata; Ha, Heterosigma akashiwo; Co, Chattonella ovata; Om, Oxyrrhis marina; Pp, Pfiesteria piscicida; Pb, Protoperidinium bipes; Gg, Gyrodinium cf. guttula. Data were obtained from Seong et al. (2006) and Jeong et al. (2008).
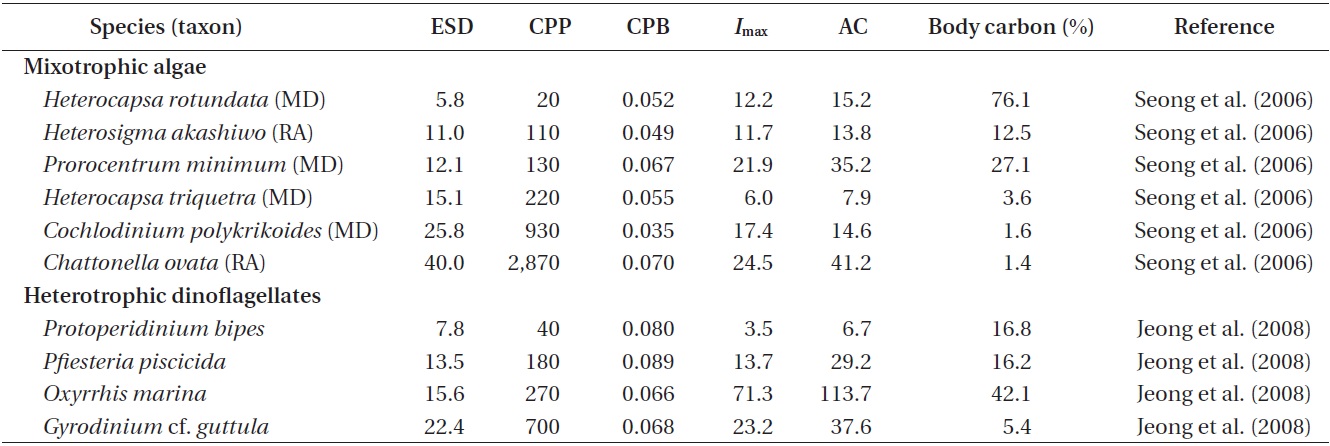
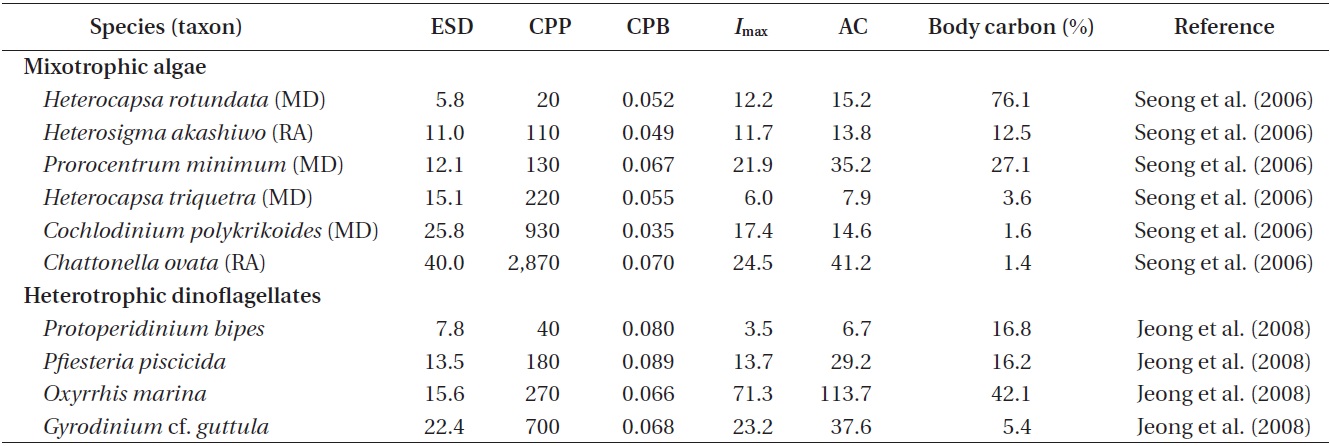
Ingestion and clearance rates measured in the laboratory. Seong et al. (2006) reported that an increase in the initial bacterial prey concentration to ca. 5 × 106 to 10 × 106 cells mL-1 led to a rapid increase in the ingestion rates by H. rotundata, H. triquetra, P. minimum, and C. polykrikoides. At higher prey concentrations, the ingestion rates increased slowly or reached saturation (Fig. 1). The maximum ingestion rates ranged from 6.0 to 21.9 cells alga-1 h-1 (Table 2, Fig. 1). Seong et al. (2006) further reported that the maximum bacterial ingestion rates of 8 red tide algae were not significantly affected by algal size, indicating that the ingestion rates of bacteria may not be determined by the size of red tide algae.
The maximum bacterial clearance rates by red tide algae were 1.0-2.3 nL alga-1 h-1 for H. rotundata, H. triquetra, P. minimum, and C. polykrikoides (Table 2). These rates are comparable with those of heterotrophic nanoflagellates (HNF, 1.0-4.0 nL alga-1 h-1) (Eccleston-Parry and Leadbeater 1994, Zubkov and Sleigh 1995), but lower than those for ciliates (50-560 nL alga-1 h-1) (Alonso et al. 2000).
Carbon acquisition from bacterial prey. Seong et al. (2006) reported that the smallest red tide alga, Heterocapsa rotundata, was able to acquire 76% of its daily body carbon intake from bacteria. The corresponding daily carbon acquisition by P. minimum was 27.1%. These data indicate that bacteria may support positive growth of small red tide dinoflagellates, with the formation of red tide patches. Carbon acquisition by H. rotundata from bacterial prey exceeds that of the heterotrophic dinoflagellate Oxyrrhis marina, which has the highest value among heterotrophic dinoflagellates (Fig. 2). Seong et al. (2006) further reported that bacteria may not support the growth of the large red tide algae Heterocapsa triquetra and Cochlodinium polykrikoides, which can obtain <4% of their daily body carbon intake from bacteria. Calculations of carbon acquisition and maximum volume-specific clearance rates by H. triquetra and C. polykrikoides indicated that bacteria are not suitable as the sole growth source for these large dinoflagellates, but may be considered as supplementary prey.
Ingestion rates measured in the field. Seong et al. (2006) reported that the mean ingestion rates of natural bacterial populations by mixotrophic red tide dinoflagellates in Korean coastal waters ranged from 1.2 to 20.6 bacteria alga-1 h-1 for H. rotundata, H. triquetra, P. minimum, P. triestinum, and C. polykrikoides. In comparison, those of HNFs and ciliates ranged from 0.7 to 39.4 bacteria HNF-1 h-1 and from 15 to 713 bacteria ciliate-1 h-1, respectively. In contrast, the grazing coefficient of natural bacterial populations by all mixotrophic red tide dinoflagellates (0.012-1.146 d-1) was significantly greater than those by all HNFs (0.008-0.196 d-1) or all ciliates (0.000-0.716 d-1).
H. rotundata / H. triquetra, P. minimum / P. triestinum, and C. polykrikoides were the most effective or the second most effective protistan predators of marine bacteria among the dominant red tide dinoflagellates, HNFs, and ciliate predators (Seong et al. 2006).
The mixotrophic red tide raphidophytes Chattonella, Heterosigma, and Fibrocapsa isolated from Korean waters are known to feed on heterotrophic bacteria (Table 2).
The maximum ingestion rates of Heterosigma akashiwo and Chattonella ovata on heterotrophic bacteria in the predators’ cultures are 11.7 and 24.5 cells alga-1 h-1, respectively (Table 2). Seong et al. (2006) reported that H. akashiwo was able to acquire 12.5% of its daily body carbon from bacteria, while C. ovata can obtain <4% of their daily body carbon. These data indicate that bacteria may support positive growth of H. akashiwo, but bacteria may be considered as supplementary prey for C. ovata.
Seong et al. (2006) reported that the ingestion rate of Heterosigma akashiwo on natural bacteria in Korean waters ranged from 2.7 to 9.0 cells alga-1h-1 and the grazing coefficient of natural bacterial populations by H. akashiwo ranged from 0.020 to 0.867 d-1. Thus, H. akashiwo may sometimes have considerable grazing impact on natural bacterial populations.
Jeong et al. (2006) and Yoo et al. (2013) reported that the abundance of the heterotrophic dinoflagellate Pfiesteria piscicida and morphologically similar heterotrophic dinoflagellates (so called Pfiesteria-like dinoflagellates) exceeded 10,000 cells mL-1 (i.e., >1,000 ng C mL-1) and caused red tides in Korean waters. P. piscicida isolated from Korean waters is known to feed on bacteria (Jeong et al. 2008). The heterotrophic dinoflagellates Oxyrrhis marina and Gyrodinium spp., isolated from Korean waters, were also able to feed on bacteria (Jeong et al. 2008). Jeong et al. (2008) further reported that the maximum bacterial ingestion rates of heterotrophic dinoflagellates were 71.3 cells dinoflagellate-1 h-1 for O. marina, 23.2 cells dinoflagellate-1 h-1 for G. cf. guttula, and 13.7 cells dinoflagellate-1 h-1 for P. piscicida. These rates are comparable with those for mixotrophic dinoflagellate (1.2-20.6 cells alga-1 h-1), raphidophytes (11.7-24.5 cells alga-1 h-1), HNFs (4-10 cells alga-1 h-1) (Eccleston-Parry and Leadbeater 1994, Zubkov and Sleigh 1995), and ciliates (150-380 cells ciliate-1 h-1) (Alonso et al. 2000). Therefore, some heterotrophic dinoflagellates may be important predators on marine bacteria (Jeong et al. 2008).
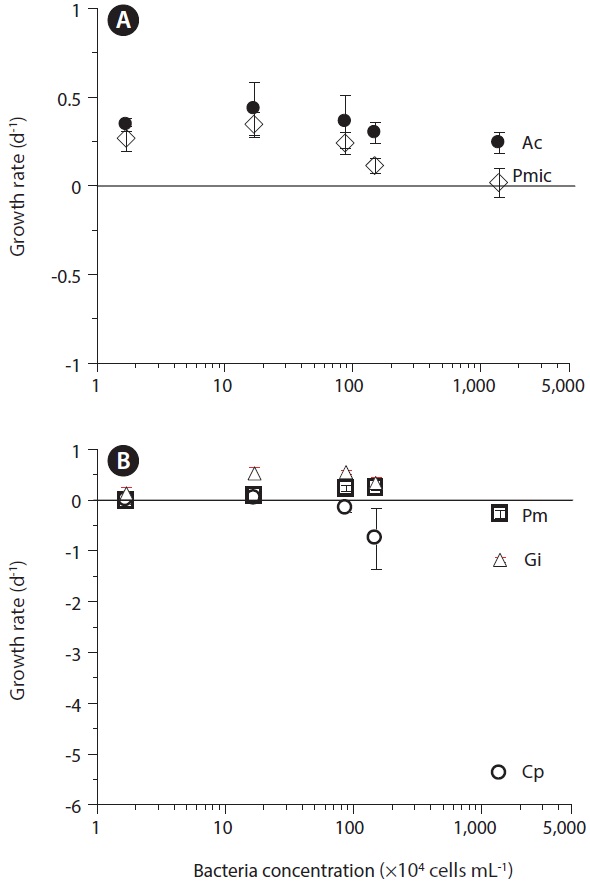
The pathogenic bacterium Vibrio parahaemolyticus is known to be a killer of dinoflagellates, and also to act as prey for red tide dinoflagellates (Seong and Jeong 2011). At V. parahaemolyticus concentrations of >1.5 × 106 cells mL-1, C. polykrikoides is a victim. At V. parahaemolyticus concentration of 1.4 × 107 cells mL-1, G. impudicum is also a victim. At V. parahaemolyticus concentrations of <1.5 × 106 cells mL-1, A. carterae, P. minimum, and P. micans are mainly grazers on V. parahaemolyticus, whereas at the higher V. parahaemolyticus concentration, they may also be victims (Fig. 3). According to these data, Seong and Jeong (2011) proposed that the roles of red tide dinoflagellates and bacteria are reversible, depending on the bacterial concentration. P. micans is able to acquire 9.2% of its daily body carbon intake (91.8 pg) from V. parahaemolyticus. Thus, V. parahaemolyticus may stimulate or partially support the growth of P. micans.
On the basis of data derived from studies of bacterial
feeding by red tide organisms, and killing of red tide organisms by algicidal bacteria, a hub of each red tide organism can be drawn (Jeong et al. 2010c). Thus, C. polykrikoides can feed on natural bacterial populations and V. parahaemolyticus, but is killed by Bacillus sp., Hahella chejuensis, and Alteromonas sp. (Table 1). Similarly, H. akashiwo can feed on natural bacterial populations, but is killed by Pseudomonas fluorescens, Micrococcus sp. LG5, and Kordia algicida OT-1. Furthermore, P. micans can feed on natural bacterial populations and V. parahaemolyticus, but is killed by Pseudomonas sp. LG2 and Micrococcus sp. LG5. Further studies are required to isolate and identify bacteria during red tides, and to determine whether the bacteria are victims or killers.
Taken together, the results of previous studies on the interactions between heterotrophic bacteria and red tide organisms indicate the following roles of each marine component in the dynamics of the other: 1) bacteria can be killers of red tide organisms; 2) bacteria can clear the body of senescent red tide organisms, by accelerating the decline of a red tide and decomposing the red tide organisms; 3) during some red tides, dominant red tide organisms may be the most effective predators of marine bacteria among protistan predators; and 4) bacteria may be too small to be ingested by filter-feeding copepods, whereas many red tide organisms are ingested by the copepods. Red tide organisms may therefore represent a link between bacteria and some zooplankters, which are unable directly to ingest bacteria.
Thus, bacteria may play diverse roles in red tide dynamics, and may even be critical factors affecting the abundance of red tide organisms in Korean waters.
Marine heterotrophic bacteria and red tide organisms can act as predators and / or prey in Korean waters. Furthermore, their roles are reversible at any time. Thus, these 2 components may co-exist by cycling materials between each other in marine ecosystems. Several methods for controlling red tides using mass-cultured algicidal bacteria have been developed. However, to evaluate the efficiency of these methods in natural environments, intensive field testing is required.













![Ingestion rates (IR; cells alga-1 h-1) of red tide dinoflagellate (A) and raphidophyte (B) on bacteria as a function of the initial prey concentration. IR values and regression curves were obtained from Seong et al. (2006). Pm, Prorocentrum minimum; Cp, Cochlodinium polykrikoides; Hr, Heterocapsa rotundata; Ht, Heterocapsa triquetra; Co, Chattonella ovate; Ha, Heterosigma akashiwo. Equations: IR = 21.9 [x/(23.9 × 106+ x)], r2 = 0.668 for Pm; IR = 17.4 [x/(26.3 × 106 + x)], r2 = 0.864 for Cp; IR = 6.0 [x/(3.2 × 106 + x)], r2 = 0.743 for Ht; IR = 11.2 [x/(9.4 × 106 + x)], r2 = 0.709 for Hr; IR = 24.5 [x/(7.2 × 106 + x)], r2 = 0.703 for Co; IR = 11.7 [x/(4.3 × 106 + x)], r2 = 0.771 for Ha.](http://oak.go.kr/repository/journal/12776/JORHBK_2013_v28n4_297_f001.jpg)