The concept of guided bone regeneration (GBR) employs a barrier membrane to prevent ingrowth of soft tissue cells but allow migration of angiogenic and osteogenic cells originating from bone marrow into bone defects (Schenk
GBR membranes can be divided into nonresorbable and bioresorbable categories. Nonresorbable membrans are composed of expanded polytetrafluoroethylene (e-PTFE) or titanium mesh. These membranes provide good biocompatibility, space maintenance for bone growth, and clinical manageability. However it is not bioresorbable, so surgical intervention for removal of membrane is required after bone regeneration. Also it can happen membrane exposure after GBR and it can often lead to local infection and GBR failure (Gotfredsen
Silk is a fibrous protein composed of sericin and fibroin that is produced by
Alkylresorcinols are natural non-isoprenoid lipids with antioxidant and antimutagenic properties that are found in various plants and microorganisms (Kozubek
We hypothesized that combining 4-HR’s antiseptic activity with silk membrane would compare favorably to collagen membrane (CM) in facilitating new bone formation in bone defects. In this study we evaluated the bone regeneration capability of silk membrane plus 3% 4-hexylresorcinol (3% 4-HR plus SM) in a rabbit calvarial defect model. New bone formation was assessed with microscopic computerized tomography (μ-CT) and histomorphometry.
Scanning electron microscope (SEM) imaging was performed with an electron microscope (Hitachi, SU-70). Attenuated total reflectance Fourier transform infrared spectroscopy (ATR-FT-IR) measurements were made with a Vertex 80 spectrometer (Bruker Optics, Germany) coupled to a microscope (Hyperion 3000, Bruker Optics, Germany) equipped with a germanium (Ge) attenuated total reflectance objective lens (ATR 20×) and a liquid nitrogen cooled mercury cadmium telluride (MCT) detector. The spectra were obtained with 256 repeated scans from 600 to 4,000 cm-1 at a resolution of 4 cm-1. X-ray diffraction (XRD) patterns of the samples were recorded with an X-ray diffractometer (PANalytical, X’Pert Pro MPD) using a Cu-Kα (λ = 1.5418 A) radiation source and collected in the range of 0-30° (2θ)
>
Animals and surgical procedures
Twenty 10 week-old New Zealand white rabbits with an average weight of 2.3 kg (range 2.0-2.5 kg) were used in this study. The study was approved by the Institutional Animal Care and Use Committee of the Gangneung-Wonju National
University, Gangneung, Korea (IACUC GWNU-2012-21).
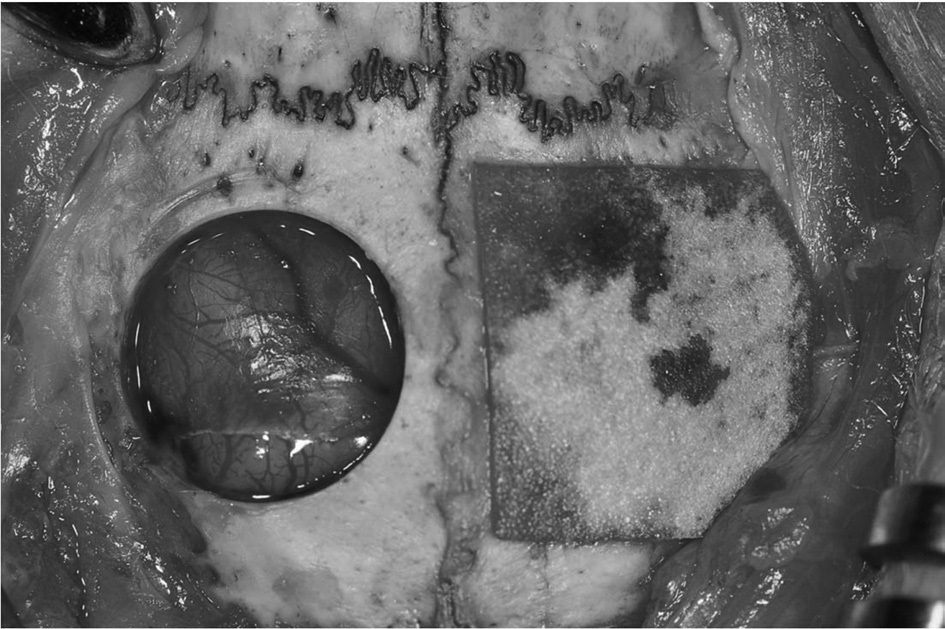
General anesthesia was administered by intramuscular injection of a combination of 0.5 mL Tiletamine and Zolazepam (125 mg/mL; Zoletil; Bayer Korea, Seoul, Korea) and 0.5 mL Xylazine hydrochloride (10 mg/kg body weight; Rompun; Bayer Korea). The cranium area was shaved and disinfected with povidoneiodine and 2% lidocaine with epinephrine (1:100,000) was applied to the cranium area. A longitudinal incision was made from the nasal bone to the occipital protuberance of the skull. Then a midline incision was made in the periosteum. Sharp subperiosteal dissection reflected the pericranium from the outer table of the cranial vault, exposing the parietal bones. A trephine bur was used under saline irrigation to create bilateral calvarial defects in the parietal bones. Two 8 mm diameter defects were created, one on each side of the midline. The calvarial defects were covered with 3% 4-HR plus SM or CM (Rapi-GuideTM, Dalim tissen, Seoul; Fig. 1). Some defects were left empty to serve as controls. Defects were randomly assigned to each group. None of the animals received the same grafts in both calvarial defects.
Following the treatment, the pericranium and skin were closed in layers with 3-0 black silk. After surgery, the rabbits received gentamycin at 1 mg/kg (Kookje, Seoul, Korea) and Pyrin at 0.5 mL/kg (Green Cross Veterinary Products, Seoul, Korea) intramuscularly 3 times daily for 3 days.
Each rabbit was individually caged and received food and water. Ten animals were killed at 4 weeks and the other ten at 8 weeks. Specimens were separated and fixed in 10% formalin. Histologic analysis was done after μ-CT analysis.
The Skyscan 1076 (SkyScan 1076; Bruker , Aartselar, Belgium) was used specimens with μ-CT. After calibration, the calvarial specimens were scanned in 0.035-mm thick sections. The scanned images were reconstructed with CT-AN 1.10 software (Bruker, Aartselaar, Belgium ). The calibrated 3-dimensional images were shown in the gross profiles of the specimens. The region of interest (ROI) was set as the initial 8.0 mm diameter round defect. A threshold level of 25% of the bone standard was set as recommended by the manufacturer. The ROI of each specimen was analyzed for bone volume (BV).
>
Histomorphometric evaluation
At 4 and 8 weeks after surgery, calvarial samples were subjected to dehydration and embedding. The specimens were decalcified with the 5% nitric acid for 2 weeks and dehydrated in ethyl alcohol and xylene. After the parietal bones were separated through the sagittal suture, they were embedded in paraffin blocks. The paraffin blocks were sliced and stained with Hematoxylin and Eosin. The section showing the widest defect area was selected, along with the cuts from 50 mm before and after. Digital images of the selected sections were made with a digital camera (DP-73; Olympus, Tokyo, Japan). The images were analyzed with a Sigma Scan Pro (SPSS, Chicago, IL). Total new bone was calculated as a percentage of the total region of the defect.
SPSS for Windows, ver. 19 (IBM Co., Armonk, NY, USA) was used for statistical analysis, and analysis of varance (ANOVA) and lease significant difference (LSD) were used as post hoc tests for comparison of the samples. The level for statistical significance was set at
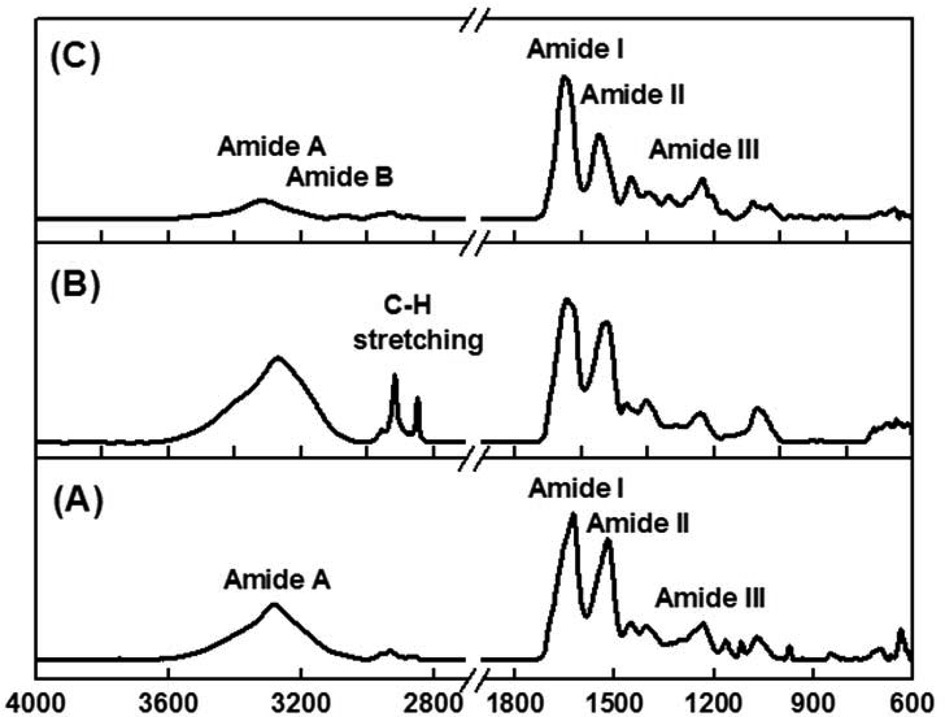
Fig. 2 shows the ATR-FT-IR spectra of SM, 3% 4-HR plus SM, and type 1 collagen. The observed spectrum of SM shows
the typical amide absorption bands in all spectral regions (Fig. 2A). The absorption bands at 1625 (amide I) and 1520 cm-1 (amide II) were attributed to a β-sheet conformation. and the absorption band at 1235 cm-1 (amide III) was attributed to a random coil structure. In the case of SM blended with the 4-HR, vibrational absorptions corresponding to pure 4-HR are seen at 2960, 2920, 2860 cm-1, which were attributed to C-H stretching vibration. Fig. 2B shows that the amide band positions and intensities were unchanged by blending with SM with 4-HR, indicating that 4-HR is well adsorbed into SM. Fig. 2C shows the absorption spectrum of type I collagen. Characteristic absorption bands appeared at 1652 (amide I), 1546 (amide II), and 1236 cm-1 (amide III). Amide A and amide B bands were found at 3320 and 3080 cm-1, respectively. The amide B band was only observed in collagen samples.
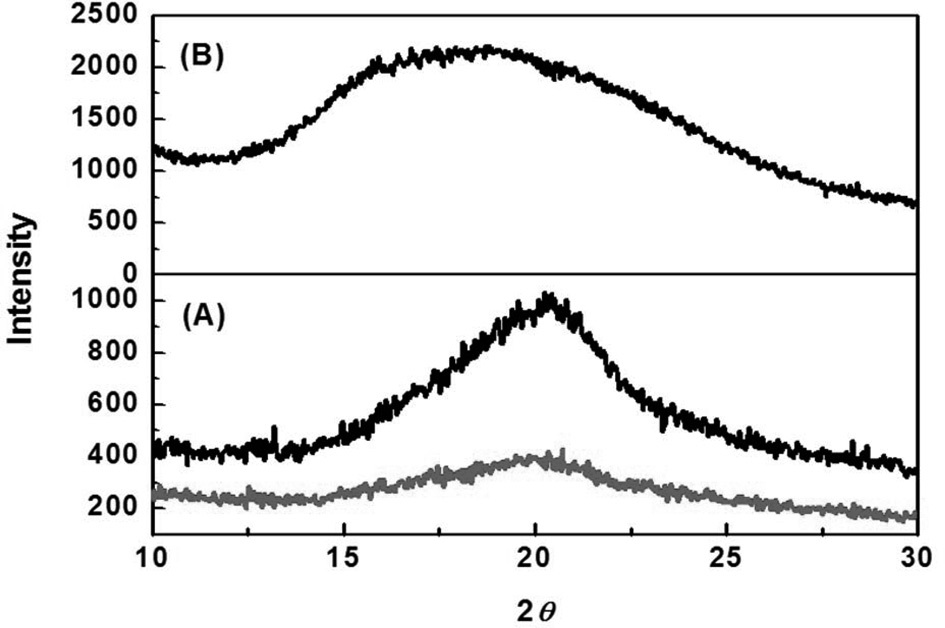
XRD patterns of SM, 3% 4-HR plus SM, and type I collagen are shown in Fig. 3. SM was characterized by the presence of the broad diffraction peak at 20° (2
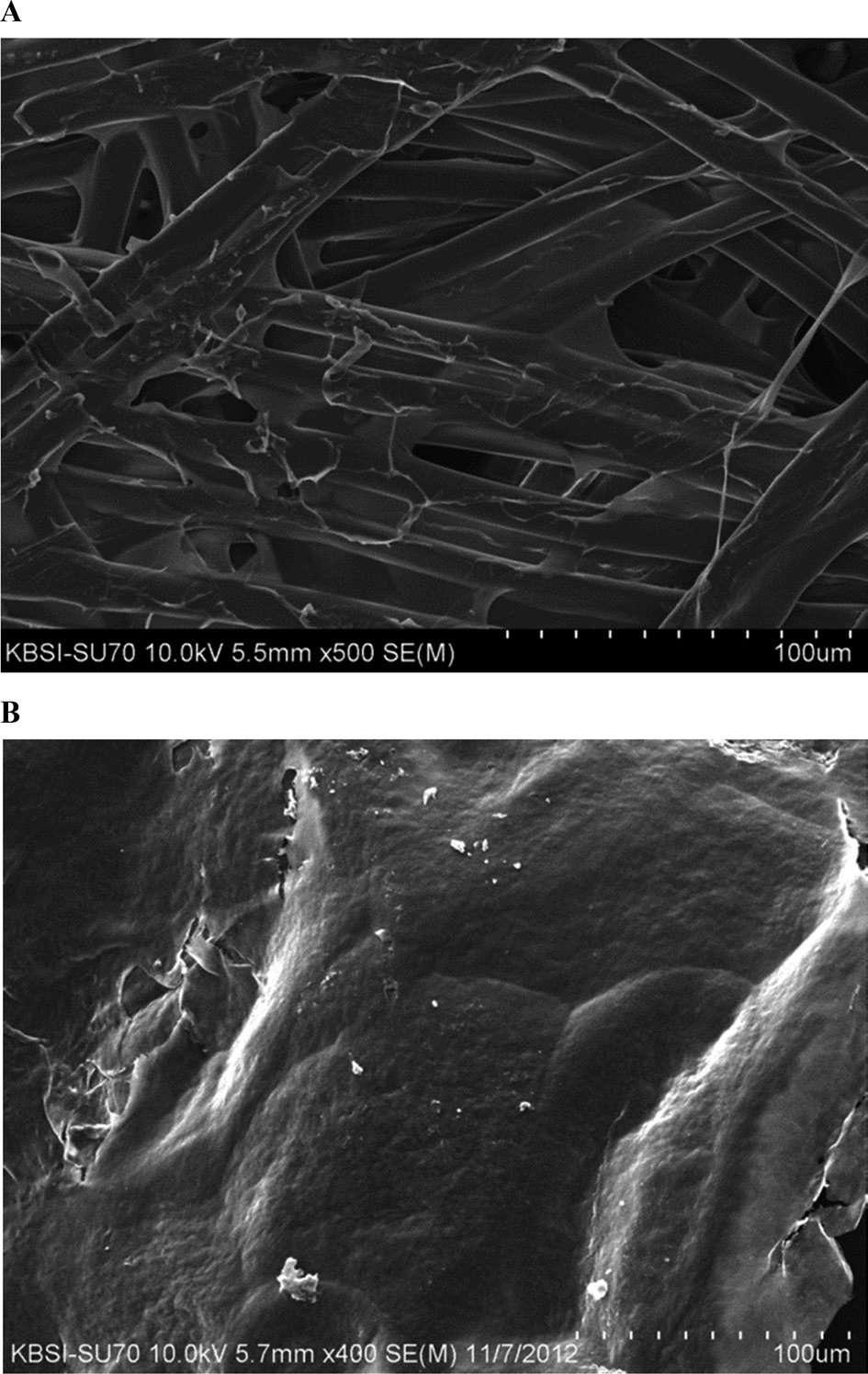
The SEM image of 3% 4-HR plus SM demonstrates the cross-linked structure of silk fiber with some glue like-material attached among the fibers (Fig. 4A). The diameter of silk fiber is approximately 100 mm. CM has a smooth surface but some
[Table 1.] Microscopic computerized tomographic analysis

Microscopic computerized tomographic analysis
regions appear to be torn (Fig. 4B).
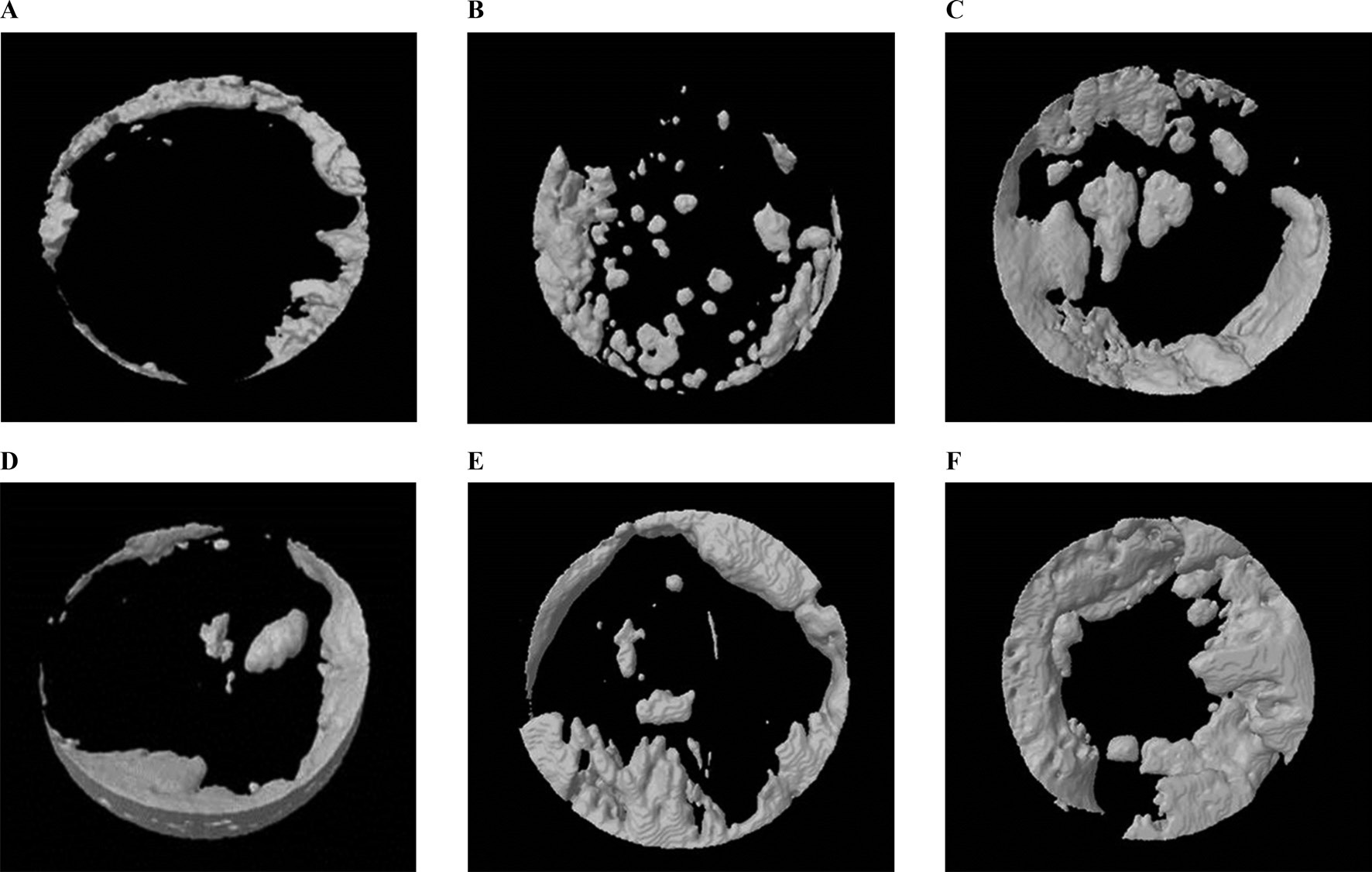
Results of the μ-CT analysis are presented in Table 1. Four weeks after surgery the volume was 12.53 ± 3.45 mm3 in the 3% 4-HR plus SM group, 9.71 ± 6.29 mm3 in the CM group, and 5.68 ± 3.27 mm3 in the unfilled group (Fig. 5). A post hoc analysis showed that the differences between the CM group and any other group were not statistically significant. However, there was a significant difference between the 3% 4-HR plus SM and unfilled groups (P=0.031).
The mean bone volume in the 3% 4-HR plus SM group was higher than the CM group. The bone volume in the 3% 4-HR plus SM group 8 weeks after surgery was 17.13 ± 6.65 mm3. That in the CM group was 16.53 ± 13.44 mm3 and the unfilled group was 18.85 ± 8.71 mm3 (Fig. 5). There was no statistically significant difference (P > 0.05).
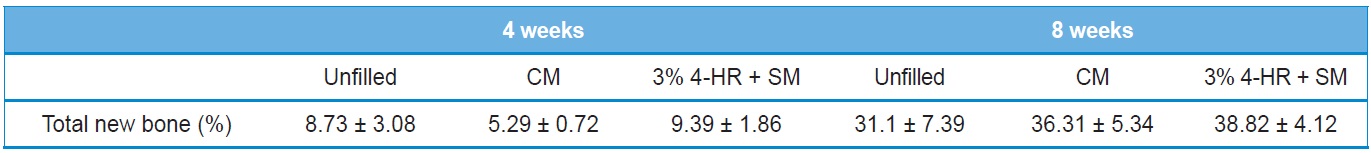
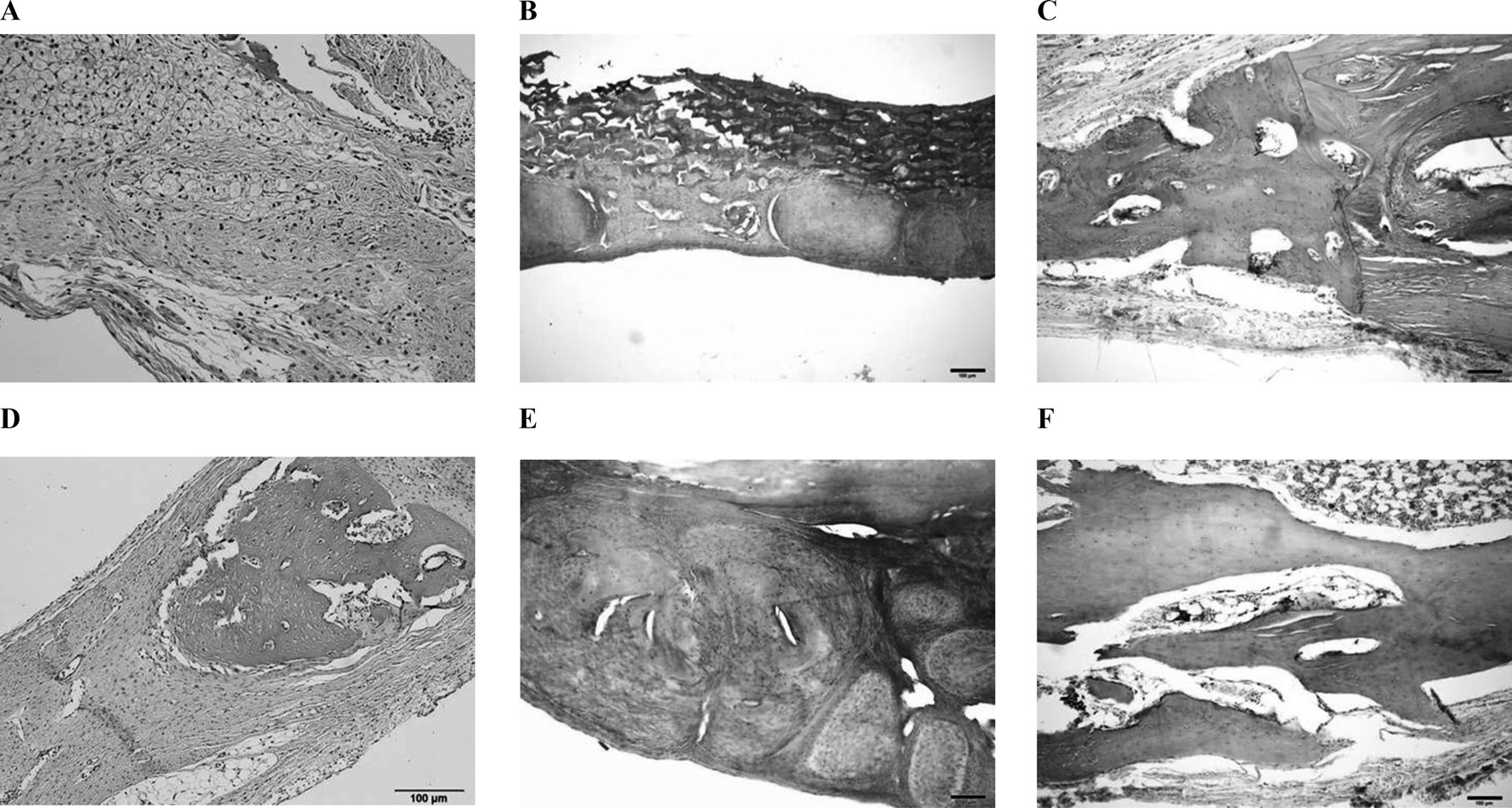
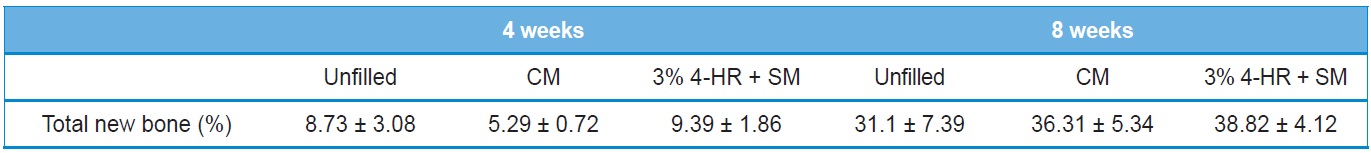
The histomorphometry results are presented in Table 2. Four weeks after surgery total new bone was 8.73 ± 3.08% in the unfilled group, 5.29 ± 0.72% in the CM group, and 9.39 ± 1.86% in the 3% 4-HR plus SM group (Fig. 6). The differences were not statistically significant (P > 0.05). Eight weeks after surgery
[Table 2.] Histomorphometric analysis
Histomorphometric analysis
total new bone was 31.1 ± 7.39% in the unfilled group, 36.31 ± 5.34% in the CM group, and 38.82 ± 4.12% in the 3% 4-HR plus SM group (Fig. 6). While these differences were not statistically significant, the average of total new bone in the 3% 4-HR plus SM group was slightly higher than the CM and unfilled groups. Well-organized lamella bony islands were formed in the 3% 4-HR plus SM group at both 4 weeks and 8 weeks (Fig. 6C and F).
Silk is used for many medical materials. In a previous study we showed that using SFM as a barrier had a positive effect on GBR in a bone defect. SFM contributed to new bone formation in a rabbit calvarial defect model compared to the uncovered control (Song
When the GBR technique is applied to the oral cavity the membrane can be exposed during healing which can lead to bacterial infection and GBR failure (Sanctis
FT-IR analysis showed that SM had a β-sheet conformation and a random coil structure. In 3% 4-HR plus SM the absorption bands of pure 4-HR were seen at 2,960, 2920, 2860 cm-1, and the amide bands of SM were maintained. XRD analysis of 3% 4-HR plus SM, showed that 4-HR did not affect to the crystallinity of SM, indicating that 4-HR was stably blended within the membrane and did not change the structure of SM.
In this study, 3% 4-HR plus SM was compared with CM. The results of μ-CT analysis 4 weeks after surgery showed greater BV in the 3% 4-HR plus SM group than in the unfilled control (P=0.031). While the mean BV of the 3% 4-HR plus SM group was greater than in the CM group, the difference was not statistically significant. Histology demonstrated that well-organized lamella bone had formed at the cutting margins of the calvarial defect in the 3% 4-HR plus SM group (Fig. 3C). Therefore, we concluded that 3% 4-HR plus SM properly maintained the space and prevented soft tissue penetration.
The results of bone regeneration in the 3% 4-HR plus SM group 4 weeks after surgery can be explained by the following observations. 4-HR has been used as an ingredient in antiseptic solutions and topical agents for infected skin and mucosa. Because 4-HR has disinfectant properties (Kim
The principle of GBR is based on guided tissue regeneration (GTR) (Retzepi
In this study, there were no statistically significant differences in BV among the 3 groups 8 weeks after surgery (Table 1). While effectiveness might be seen in the initial healing period, the effect of 4-HR on bone formation does not persist for 8 weeks. Further study is required to understand the 8-week results.
In conclusion, μ-CT analysis and histologic analysis showed that the 3% 4-HR plus SM group had more bone formation 4 weeks after surgery when compared to that of CM and unfilled control groups. 3% 4-HR plus SM showed successful early bone regeneration in a rabbit calvarial defect model.